Introduction
The evolution of biomaterials adopted for orthopaedic use might well be described as slow and incremental. There are a number of contributing reasons, but the conservative nature of orthopaedic surgeons and the effects of the 510(k) approval process on the development of novel materials and PMAs that require clinical trials remain major factors. It could be argued that advances in hip and knee implants have been minor since the days of John Charnley, since both stainless steel and ultra high molecular weight polyethylene are still widely employed in orthopaedic implants 50 years after their introduction. Of course, the highly successful clinical outcomes of hip and knee arthroplasty attests to excellence in the original Charnley design, but it must be recognized that current orthopaedic materials are sub-optimal from several standpoints. In this article, we will address how aerospace materials, particularly composites, might have direct applications to orthopaedic practice due to advanced material properties.
Major Drawbacks to Current Orthopaedic Materials
Although complications in arthroplasty such as infection and operative error have fallen below five percent for most procedures,[1] three areas of adverse outcomes are well recognized in orthopaedic science:
Wear debris-induced osteolysis. Joint arthroplasty requires the replacement of an articulating joint with a prosthesis that enables movement through a load-bearing surface. All current bearing surfaces are subject to the generation of wear during use, and the current materials in debris form have proven provocative of macrophage recruitment and attendant chronic inflammation. This outcome ultimately drives osteoclast-mediated bone resorption that leads to loss of fixation of the prosthesis commonly termed aseptic loosening.[2] We believe that low friction aerospace polymers and composites such as PEEK (polyetheretherketone) may provide superior bearing surfaces that improve the performance and life expectancy of joint prostheses.
Stress shielding. The quality of bone and its structural stability are dependent upon force applied to the skeleton during normal activities (Wolffe’s Law). The implantation of metal femoral and tibial components during arthroplasty results in abnormal distribution of forces to the bone tissues, due to the stiffness of the prosthesis components. This attenuation of the normal bone loading during motion results in reduced osteoblast activity and subsequent regional bone loss in tissues adjacent to the prosthetic components. Aerospace engineering suggests that composites can be matched to the modulus of bone, allowing natural force transmission from the implant to the bone resulting in the alleviation of bone loss due to stress shielding.
Material biocompatibility. Recent reports of adverse reactions to metal-on-metal (MoM) hip implants have alerted patients and surgeons to the phenomenon of metal hypersensitivity. The release of metallic ions from orthopaedic implants (due to both bearing wear and corrosive action on metals) has been reported to result in hypersensitive responses to nickel, cobalt and chrome in 30% of patients with well functioning MoM implants (vs. 15% in normal controls), and hypersensitivity can reach as high as 66% in patients with failing prostheses.[3] The mechanism for the development of metal hypersensitivity is well-recognized by clinical immunologists, and this form of allergy is inevitable in a large subset of orthopaedic patients. The minimal biological response to many aerospace materials indicates a high level of biocompatibility appropriate for use in orthopaedic procedures.
Several studies have illustrated the potential for composite materials to provide successful adaptation to orthopaedic implantation, but some difficulties have arisen in clinical acceptance of these designs.[4,5] Aerospace technology may provide the key to the advancement of composite materials, since several decades have been devoted to material performance in harsh environments and extreme mechanical loading. It is also recognized that composites can provide advantages over metals in instruments and equipment used over the vast range of delivery of orthopaedic care, and this philosophy is illustrated in projects under development at the National Center of Innovation for Biomaterials in Orthopaedic Research (nCiBOR). The particular advantages of an improved strength to weight ratio and radiolucency during imaging are major motivating factors for innovation using composite based materials. Challenges arise due to the limited knowledge of long-term performance of these materials. We describe three applications of aerospace materials under development of orthopaedic applications.
1. Composite use for surgical instruments. Numerous surgical instruments are employed in the practice of orthopaedic surgery, including surgical bone awls, bone-hold forceps, bone tampers and surgical elevators, osteotomes and surgical retractors. Traditionally, these have been manufactured using stainless steel. Since intra-operative fluoroscopy is often desirable during surgery, and essential during many trauma procedures, multiple rounds of removal and reinsertion of all instruments may be required to avoid interference with the interpretation of x-ray imaging. Instrument removal and replacement contributes to extended operating time and could contribute to increased mortality and morbidity. Further, the use of steel instruments in close proximity to hip and knee bearing surfaces runs the risk of surface scratching, even at a microscopic level. Scratches on bearing surfaces can have a devastating effect on wear of the implant biomaterials, with a subsequent reduction of implant life and the concomitant problem of debris-induced osteolysis. CIBOR is developing composite surgical instruments that take advantage of weight reduction, radiolucency and non-marring surfaces that aerospace composite materials provide. A range of issues arise to implement this innovation:
- The identification of appropriate x-ray compliant composites appropriate for the development of x-ray transparent or translucent instruments.[6]
- Development of design criteria that balance fluoroscopy interference and instrument biomechanics required for surgical procedures.
- Retention of mechanical stability and bioburden removal for composite instruments subsequent to multiple cycles of autoclave sterilization.
- Identification of potential modes of failure for instruments compared with stainless steel equivalents, and determination of the potential risk for creation of composite shards that might arise from instrument breakage or damage by cutting instruments.
Findings to date indicate that composite materials can be identified that provide either complete radiolucency or engineered to exhibit a “ghosting” effect to allow accurate positioning during fluoroscopic procedures. We have observed significant variations between typical aerospace composites when subjected to repeated autoclaving and mechanical testing, but data is now available to suggest that composite instruments should provide a useful lifetime comparable to their stainless steel counterparts. Short term in vivo tests of inflammation provocation using an animal model developed in our labs have demonstrated that risks due to composite shards and smaller debris particles are within an acceptable material range.
2. Composite applications for rapid fixation devices. Battlefield injuries present a complexity of medical problems in a hostile environment, often at considerable distance from trauma care. Orthopaedic injuries constitute a majority of the combat casualties in recent U.S. military conflicts, and 16% of cases involve segmental bone defects or complex fractures.[7] These injuries are invariably complicated by open wounds, and avoiding increased morbidity and mortality requires rapid and rough patient transportation under difficult conditions. The severity of these injuries in conjunction with demanding logistical considerations contribute to a high probability of vascular and nerve damage due to lack of bone fixation. Medical treatment outcomes for battlefield injuries could be improved using a lightweight composite fixation device that provides limb stability and protection from harsh environmental conditions. The development of modern body armor has resulted in a pattern of battlefield injuries that concentrate trauma to the extremities. This is particularly apparent for IED injuries, which result in extensive tissue damage, high risk of contamination and a requirement for orthopaedic treatment in over half of the casualties.[8,9] Inadequate fixation of unstable fractures can result in further damage to the vasculature and nervous system during transport, which may ultimately result in amputation of an injured limb considered to have reasonable salvage potential.[10] CIBOR is conducting research to develop and design concepts, techniques and materials to improve survivability and ensure better medical treatment outcomes in pre-hospital settings by applying aerospace composite technologies to military medical stabilization devices. The product will be a fast setting composite stabilization device that will initially enable shape manipulation and then harden to create a stiff, protective custom-shaped fracture fixation device. This lightweight, compact, portable device will enable quick fracture stabilization in the field. It will conform closely to the shape of the injury site while providing support for improved patient comfort, thereby reducing risk of soft tissue damage during transport. In addition to improved fixation, it will also improve triage decisions with low interference of x-ray imaging.
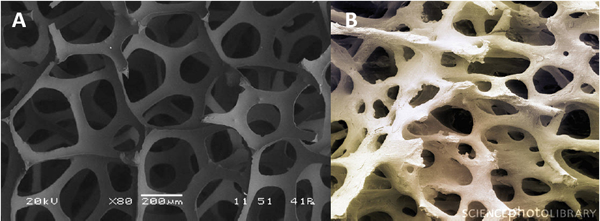
3. Carbon foam development as a bone graft scaffold. There is a continuing need for bone grafts and bone graft substitute (BGS) materials to exploit the self-regenerating capacity of bone to enhance both the rate and extent of bone defect repair. At present, BGS options for patients and practitioners include anorganic materials, demineralized bone matrix, nonproprietary allografts, stem cells and recombinant growth. It is anticipated that graft-facilitated orthopaedic procedures will continue to increase at least through 2015 as a result of an aging demographic. Unfortunately, multiple clinical and market forces working to limit the range of surgical options have arisen for many orthopedic procedures. These include a trend toward strict cost controls necessitated by changing third-party payment policies, an innovation-attenuating trend precipitated by declining numbers of “elective” surgeries, and negative publicity attending adverse events suspected of being caused by the use of InFuse (Medtronic; BMP-2) in spinal fusion procedures as well as in off-label uses.[11] Should the availability of recombinant cytokines be reduced either through increased contraindications or proven to be causative of serious disease or injury, an as-yet undefined gap in patient care would be the likely outcome. Work at CIBOR has focused upon providing a proof-of-concept for the use of carbon foam materials as BGS alternatives for orthopaedic procedures. A number of distinct carbon foam materials have been independently developed for various industrial applications based upon desirable properties that include high heat transfer, electrical conductivity, low chemical reactivity and low coefficient of expansion. Significantly, these same functional requirements have culminated in new materials that resemble cancellous bone with respect to pore size, pore structure, surface area as depicted in Exhibit 1, and in some cases, the relationship of the mechanical properties of trabecular bone can be approximated.


Exhibit 1. SEM images of carbon foam and cancellous bone samples (80X).
Initial studies of biocompatibility using the in vitro L929 mouse fibroblast cell assay system demonstrated no cytotoxicity, and stromal cells exhibited excellent attachment and spreading onto vitreous carbon surfaces. Carbon foam surfaces also support BMP-2-mediated differentiation of cells as demonstrated by alkaline phosphatase induction by a mouse mesenchymal cell line (Exhibit 2A) and matrix mineralization by a mouse osteoblast cell line (Exhibit 2B).


Exhibit 2. Carbon foam supports BMP-2-mediated alkaline phosphatase and ECM mineralization of muscle-derived mesenchynmal cells and osteoblasts.
A critical defect model was employed to evaluate carbon materials in a 6mm femoral defect in a rat femur. The implanted cylinders coated with BMP-2 were fixed using a metal pin (Exhibit 3A), and the repair process analyzed by microCT (Exhibit 3B) and histology (Exhibit 3C) 90 days after implantation. Virtually complete bridging bone was seen in the BMP-treated carbon implants by both analytical criteria showing a profile and density of new bone comparable to the unimplanted contralateral femur. The remodeling of the scaffold with removal of the carbon biomaterial from the repair site suggests an ideal profile for an advanced bone void filler.
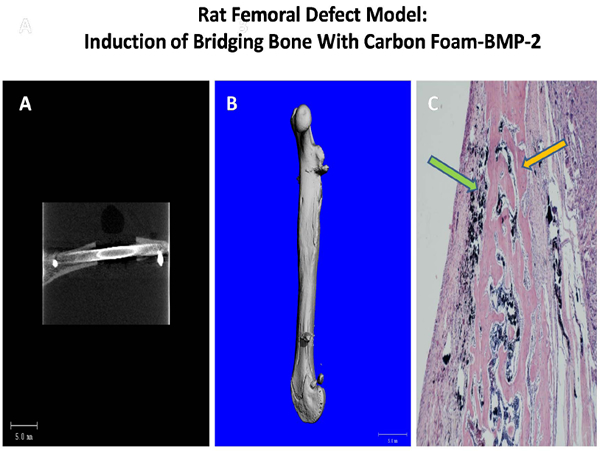

Exhibit 3. Rat femoral defect model employing BMP-2-coated carbon foam depicting post operative view of pin fixation (A), microCT image of mineralization at 90 days (B) and corresponding histological image (C) showing new bone (orange arrow) surrounding carbon scaffolds (green arrow).
Summary
Aerospace materials are showing promising initial biomaterial properties for use in orthopaedic applications. Compatibility studies using both in vitro and in vivo techniques suggest an excellent inflammatory and immune profile for many composites in a range of applications. Strength to weight ratios indicate significant advantages over metals for bone replacement and repair techniques, and reduced interference using imaging techniques should provide distinct advantages for these biomaterials in future orthopaedic applications.
References
(1) Katz JN, Bierbaum BE, Losina E. Case mix and outcomes of total knee replacement in orthopaedic specialty hospitals. Medical Care 2008; 46:476-480.
(2) Bauer TW, Schils J. The pathology of total joint arthroplasty.II. Mechanisms of implant failure. Skeletal Radiol 1999; 28(9):483-497.
(3) Jacobs JJ, Goodman SB, Sumner DR, Hallab N. Biologic Response to Orthopaedic Implants. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic Basic Science. 2nd ed. AAOS; 2000. 401-426.
(4) Akhavan S, Matthiesen MM, Schulte L, Penoyar T, Kraay MJ, Rimnac CM et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem 11037. The Journal Of Bone And Joint Surgery American Volume 2006; 88:1308-1314.
(5) Field RE, Cronin MD, Singh PJ, Burtenshaw C, Rushton N. Bone remodeling around the Cambridge cup: a DEXA study of 50 hips over 2 years 11036. Acta Orthopaedica 2006; 77:726-732.
(6) Baidya KP, Ramakrishna S, Rahman M, Ritchie A. Quantitative radiographic analysis of fiber reinforced polymer composites. J Biomater Appl 2001; 15:279-289.
(7) Lin DL, Kirk KL, Murphy KP, McHale KA, Doukas WC. Evaluation of orthopaedic injuries in Operation Enduring Freedom. J Orthop Trauma 2004; 18(8 Suppl):S48-S53.
(8) Camuso MR. Far-forward fracture stabilization: external fixation versus splinting. J Am Acad Orthop Surg 2006; 14(10 Spec No.):S118-S123.
(9) Ryan JM, Cooper GJ, Haywood IR, Milner SM. Field surgery on a future conventional battlefield: strategy and wound management. Ann R Coll Surg Engl 1991; 73(1):13-20.
(10) Cohen A, Baldwin JN, Grant RN. Problems in the management of battlefield vascular injuries. Am J Surg 1969; 118(4):526-530.
(11) Lad SP, Nathan JK, Boakye M. Trends in the use of bone morphogenetic protein as a substitute to autologous iliac crest bone grafting for spinal fusion procedures in the United States. Spine 2011; 36:E274-E281.
Paul H. Wooley, Ph.D. is Chief Scientific Officer for the National Center of Innovation for Biomaterials in Orthopaedic Research (nCiBOR) and Director of the Orthopaedic Research Institute in Wichita, Kansas. Please reach Dr. Wooley at Paul.Wooley@viachristi.org.
National Center of Innovation for Biomaterials in Orthopaedic Research
www.ncibor.net




