Nitinol (NiTi) is widely used in medical devices due to its superior superelasticity, shape memory effect, low stiffness, damping, biocompatibility and corrosion resistance. Major applications of Nitinol include stents, guidewires, orthodontic archwires and bone staples. The functional properties of Nitinol medical device components are sensitive to composition and production thermal gradients. Subtle changes in chemistry and heat treatment can lead to large variations in austenite finish (Af) temperature of the finished component.
Conventional subtractive manufacturing of Nitinol has several limitations, for instance, machining associated work hardening. This makes additive manufacturing (AM) of Nitinol an appealing alternative to traditional manufacturing. Additive manufacturing is effective in fabricating complex device geometries with pre-designed porosity, homogeneous composition and desirable properties compared to traditional manufacturing techniques. The additive manufacturing process can achieve structures with high density and near net shape, requiring very little or no post-processing.
However, the effective translation of shape memory and superelastic properties create challenges for additively manufactured Nitinol components. This is primarily due to the influence of alloy chemistry on functional properties, creating a barrier to large scale production of 3D-printed Nitinol devices. It is, therefore, critical to understand the interplay of the atomization process along with printing parameters on the additively manufactured Nitinol component.
Carpenter Technology and their branch of 3D printing experts, Carpenter Additive, carried out a study to create a systematic framework to optimize additive manufacturing for Nitinol.
The study focused on three factors for success:
- Stringent control of Nitinol chemistry throughout the AM lifecycle, from feedstock to finished part
- Optimized printing parameter sets to enable effective control over shape memory and superelastic properties
- Fully dense near net shape Nitinol components to eliminate additional post-processing steps
Alloy Chemistry
Subtle changes in the alloy composition of Nitinol can significantly alter phase transformation temperatures. For instance, a 1% increase in Ni content decreases the transformation temperature by around 100°C, along with an associated increase in the austenitic yield strength. Such extreme sensitivities of austenite finish temperatures to the base chemistry make it difficult to produce Nitinol alloys in powder form for additive manufacturing.
During atomization, the feedstock is exposed to extremely high temperatures that typically lead to the evaporation of nickel, while a loss of the element has also been observed during the 3D printing process due to the exposure to the high energy laser beam. The use of pressurized inert gas during atomization, as with the electrode inert gas atomized (EIGA) process used by Carpenter Additive to produce Nitinol powder, helps to control and maintain a higher level of Ni content in the powder post-atomization.
Parameter Optimization
Carpenter Additive collaborated to develop a unique proprietary framework for the optimization of printing parameters. For a particular chemistry of NiTi, the initial data is obtained from finite element analysis of the melt pool thermal model for NiTi alloy systems and used to develop several initial parameter sets, combining variations of laser power vs. velocity, for a matrix of single-track printing trials. The results from these trials are used to further refine the model to eliminate those parameter sets that lead to artifacts such as lack of fusion, keyholing or balling. This exercise typically provides a region of parameter sets that result in optimal single-track builds (See Exhibit 1.) and facilitates the determination of boundary conditions of the processing parameters. The model then incorporates hatch spacing as an additional criterion to construct guides for parameter selection of bulk sample fabrication. With an optimal combination of laser power, velocity, and hatch spacing, Carpenter Additive has achieved ~99.9% dense 3D printed NiTi components.
Exhibit 1: Single-Track Print Tests
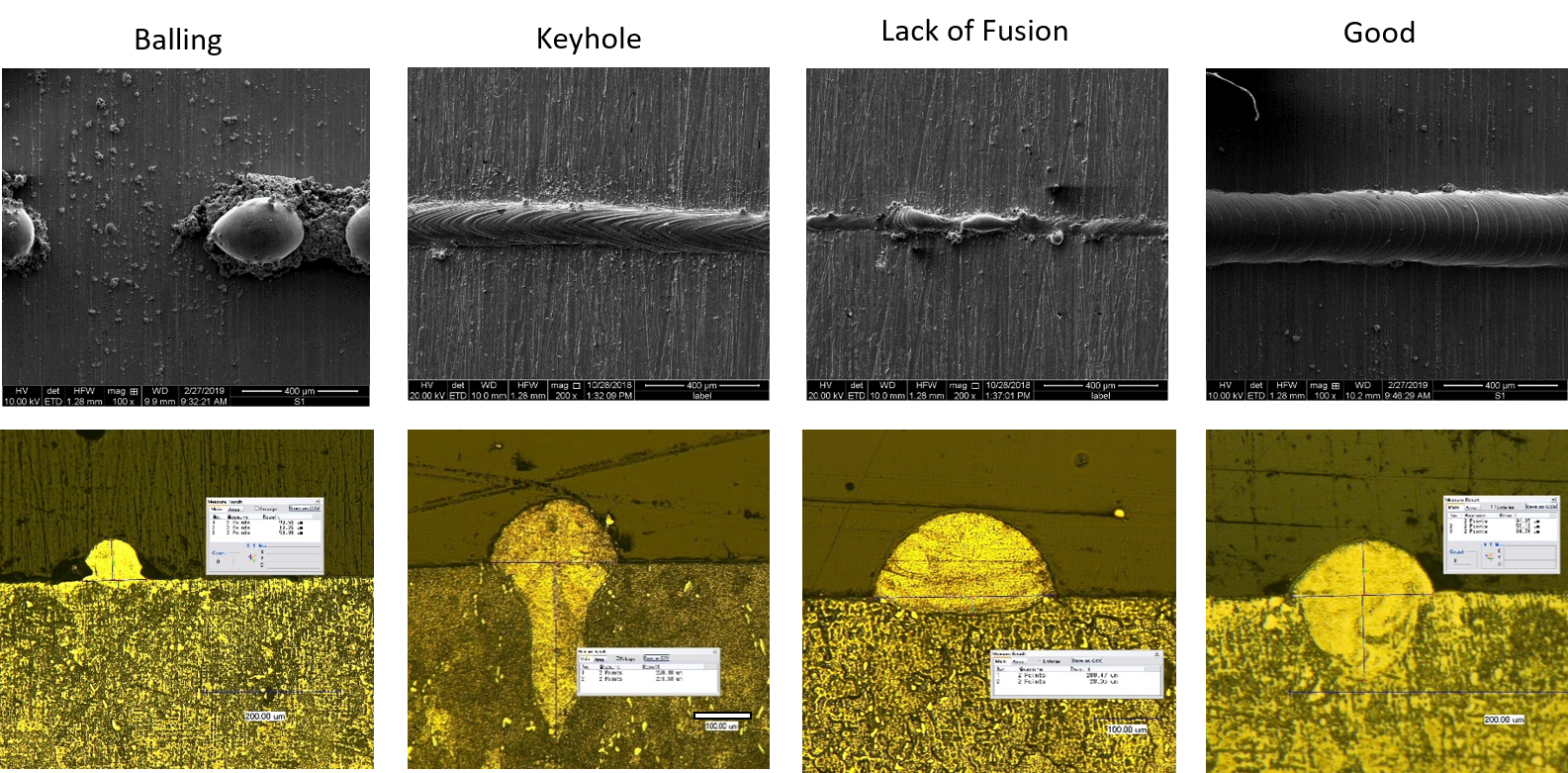

The framework optimization model is universal. The systematic approach to determine optimum build parameters can be utilized for any Nitinol chemistry, accelerating initial development and leading to significant cost savings by reducing trial and error with repeated Nitinol builds.
Process Optimization
Additive manufacturing enables production at near-net shapes to minimize or in some cases eliminate post-processing steps. Nitinol has a significant work-hardening rate, often leading to challenges in the fabrication of components through conventional machining methods. NiTi alloys also cause significant tool wear, so the reduction of secondary machining operations improves production efficiency.
By producing fully dense, near-net shape geometries directly through 3D printing, manufacturers can reduce complications that arise from traditional machining of Nitinol components.
Successful Shape Memory and Superelastic Effect
Using this systematic approach, Carpenter Additive printed NiTi samples of varying compositions – samples exhibiting shape memory and superelasticity at room temperature. These samples were subsequently subjected to cyclic tension testing at room temperature. Initial results from the shape memory composition show ~6% of recoverable strain upon heating. The superelastic NiTi also showed reversible strain of ~6%. More work on this study is currently underway. Carpenter Additive used an in-house produced Nitinol composition to demonstrate the shape memory and superelastic effect in a 3D-printed Nitinol bone staple for orthopedic applications.
Initial results of the study are available here.
Dr. Gaurav Lalwani is the Global Medical Applications Engineering Lead at Carpenter Technology Corporation, where he manages the technology portfolio and new product development strategy for the medical business unit. He is currently engaged in the development of novel wrought and additive solutions for the medical device industry. With a MS and Ph.D. in Biomedical Engineering from Stony Brook University with a focus on materials science, tissue engineering and regenerative medicine, Dr. Lalwani was the co-founder of Millennial Material & Devices and Vice President of Research for Theragnostic Technologies Inc., seed stage biomedical startups. He is an active member of ASTM, MRS, BMES, ECS and other societies and holds a keen interest in promoting inclusive education and food safety by supporting and advising organizations focused on social entrepreneurship.