High-performance thermoplastics are enjoying strong growth as a replacement for metal in a broad range of medical and healthcare applications. These high-performance materials are known for their mechanical properties and are proven in their unique ability to cut system costs through lower production cost and a reduction in the number of secondary operations, such as machining. Metal-to-plastics conversion also provides additional benefits including weight reduction, chemical and corrosion resistance, design freedom, and improved functionality of the final device.
Numerous trends in the healthcare industry favor the use of high-performance thermoplastics in a range of applications including instruments and devices, sterilization cases/trays and medical equipment. These trends are focused on cost reduction, but also include prevention of infectious disease, practitioner comfort, product differentiation and improved logistics.
The Economic Advantage
In economic terms, high-performance thermoplastics deliver significant system cost reductions. And while it’s difficult to cite a firm cost number, since each part has its own variables (weight, design, etc.), it can be estimated that the manufacturing cost can be reduced by up to 90 percent when replacing metal with injection molded plastics. This economic advantage will come primarily from the more cost-effective manufacturing process and reduced number of manufacturing steps to produce the final part.
High-performance thermoplastics can provide a weight reduction of up to 80 percent compared to surgical stainless steel. This enormous weight reduction helps reduce practitioner fatigue during long procedures and promotes greater precision. Additionally, the use of high-performance plastics allows greater design flexibility and improved ergonomics through the integration of functions such as silicone over molding to make soft-touch parts that facilitate the practitioner’s work. Such a weight reduction, particularly in surgical instruments and sterilization cases/trays, also facilitates handling, particularly during disinfecting and sterilization processes, and reduces the weight that nurses and other hospital personnel must carry, thus improving worker safety.
Some high-performance thermoplastics also offer excellent compatibility with commercial sterilization techniques, thus helping to prevent the spread of infectious disease. In material selection, end users and processors must consider the type of sterilization methods that will be used and number of cycles, along with the cleaning agents and disinfectants, because these will dictate the materials that can be used for the application.For example, only a handful ofhigh-performance plastics such as polyphenylsulfone (PPSU), polyaryletherketone (PAEK) and polyetheretherketone (PEEK) can withstand over 1,000 steam sterilization cycles without any adverse effect on mechanical properties.
Medical device manufacturers can also achieve product differentiationby designing with high-performance plastics. For example, products molded of colored resins allow for quick identification of the instruments (type and/or size) during a procedure and also permit the use of a manufacturer’s branding or trademark. Because plastics are integrally colored, molded parts will retain the same color after scratching and marring, unlike painted parts that can be damaged. Decorating options,, such as pad printing and laser etching are also possible for identification.
In terms of logistics, radiofrequency identification (RFID) technology for plastic parts is under evaluation and is already used in some segments of the healthcare industry. Plastics in general will allow RFID technology to function perfectly, while aluminum will create strong interference, making its use difficult. RFID tracking combined with high-performance plastics is already used successfully in other industries such as aircraft catering trolleys. For RFID applications, cost-effective high-performance plastics with strong mechanical properties are being targeted for multiple-use applications as a replacement for aluminum.
Understanding Plastic Design and Material Selection
Resin suppliers continue to develop new materials with improved mechanical properties to facilitate the replacement of metal in healthcare applications. However, perceptions play a key role in metal-to-plastics conversion, since most designers still tend to think in terms of direct part-to-part replacement. As a result, development of plastic parts can require several cycles of “make it and break it” which add both cost and length to the overall development time. To shorten that cycle, designers need to understand material properties and redesign accordingly. High-performance plastics don’t have the same mechanical properties as metals, so part design will need to be adapted to plastics, paying special attention to part thickness, equivalent stiffness, use of ribs and radius. Designers must also manage tolerances and make use of computer-aided engineering (CAE).
Material selection is critical in order to avoid under- or over-specifying the proper material requirements. The application will fail if under-specified, while costs will run too high if the product is over-specified. When considering thermoplastics over metal, several criteria must be evaluated, including:
- biocompatibility
- primary function of the instrument or device
- product service life
- size of production run
The first step in material selection for healthcare applications is assessing biocompatibility requirements and determining whether the part will be in contact with body fluids and for how long. Thebiocompatibility requirementswill define the first group of materials to evaluate for the metal to plastic conversion. Every country has its own legislation, but a standardized test method, ISO 10993, is followed by the healthcare industry in the U.S. and Europe.
Another key factor in material selection is determining the primary function of the instrument or device. For example, devices or instruments subjected to considerable mechanical forces such as traction, compression and shear will typically need to be molded from highly reinforced (glass or carbon fiber filled) plastics such as PEEK. Meanwhile, applications that require greater dimensional stability and tighter tolerances will lean more towards unreinforced engineering plastics such as PPSU.
Understanding the device’s service life and its single-use or reusable status will again narrow the potential material candidates. A key factor to consider will be the sterilization method and how it will affect material properties, and thus the device performance. This is important because a material often times is selected for one specific property, and sterilization can often affect that performance attribute, particularly in reusable applications. An often used method is steam sterilization, which exposes devices to steam at 134°C for approximately 18 minutes. This amount of heat in a steam environment is harmful to many plastics, particularly after repeated exposures. But high-performance plastics such as PPSU, PAEK and PEEK will withstand repeated sterilizations without adversely affecting performance. Competitive materials such as polyamides (nylons) suffer a strong decrease in mechanical performance in just a few sterilizations and will not be recommended for use in instruments or devices that require repeated steam sterilization. Another important consideration is that these high-performance plastics require resistance to highly aggressive cleaning and disinfecting agents that are applied to the instruments or devices before sterilization.
The production run, too, will play a key role in the design of the medical instrument or device and the selection of the manufacturing method. The healthcare industry is moving away from multiple designs for a single product due to cost pressures, resulting in growing use of standardized designs. High-performance plastics can be converted via several manufacturing methods such as thermoforming, extrusion and injection molding. For example, thermoforming and extrusion are geared to more small-volume production, while injection molding is targeted for high-volume manufacturing. Injection molding is generally regarded as the most economical method for production starting at 5,000-10,000 parts per year and up.
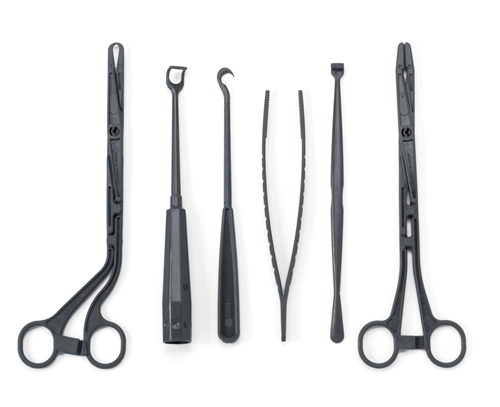
Exhibit 1 shows a successful metal to plastic conversion of a set of surgical instruments. In the past, these instruments were manufactured from stainless steel and were reusable. Currently they are made of glass reinforced PARA, and are single-use. The opportunity of metal to plastic conversion is not limited to general surgical instruments, and can also be translated to procedure specific instruments.
Exhibit 1: Results of successful metal to plastic conversion, surgical instruments


The demand for high-performance plastics as a metal replacement option in medical devices and instruments will continue to grow. Plastics’ wide array of combinations and moldability create a broad palette of design opportunities that can reduce cost, lower weight, create product differentiation and dramatically improve overall performance. Designing with plastics requires different considerations than when designing with metal, but OEMs and processors can look to suppliers of engineering resins for help in material selection and design to ensure the development of a successful new application.
Margarita Alonso is the Global Market Manager for Healthcare at Solvay Advanced Polymers. She holds a degree in Chemical Engineering from Ecole Européenne de Chimie, Polymères et Matériaux de Strasbourg (E.C.P.M.) in France, as well as a degree in Chemistry from Universitat de Ciències Químiques de Barcelona, Spain.She joined Solvay in 2005 as Business Development Representative for France for the non automotive market. Since November 2010, she has supported Solvay’s Healthcare team for the non implant-related activities. Her prior positions include Development & Marketing Manager, Asahi Thermofil France and Technical & Marketing Representative, Polymerland Guzman.
Solvay Advanced Polymers, LLC, produces more plastics with more performance than any other company in the world. This gives design engineers worldwide more ways to solve top design challenges in automotive, medical, electronics, aerospace and other demanding industries. Learn more at www.solvayadvancedpolymers.com.
Solvay is an international industrial group active in Chemistry. It offers a broad range of products and solutions that contribute to improving quality of life. The Group is headquartered in Brussels and employs 17,000 people in over 40 countries. In 2009, its consolidated sales amounted to EUR 8.5 billion.




