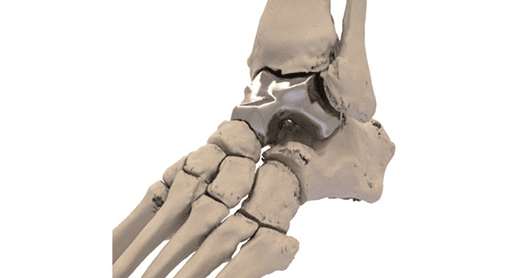
FDA announced immediate humanitarian use for the world’s first patient-tailored talus replacement implant. The Patient Specific Talus Spacer by Additive Orthopaedics is a 3D-printed implant used to alleviate the impact of avascular necrosis of the talus, a rare but debilitating condition affecting the ankle joint bone located at the juncture where the foot and leg connect.
Surgeons use computed tomography imaging to first capture a three-dimensional view of a patient’s ankle and foot, and then construct the implant to these anatomical specifications using a cobalt chromium alloy. Surgeons then remove the talus bone and install the implant during a replacement procedure.
Avascular necrosis occurs when blood flow is cut off to bone tissue due to an injury or other damage. When the dead (or “necrotized”) tissue is located in a joint, the cartilage can also wear down; in the ankle especially, this can cause arthritis, pain and in some serious cases, the talus bone can lose stability.
“Avascular necrosis of the talus is extremely painful and debilitating for these patients,” said Greg Kowalczyk, President of Additive Orthopaedics. “Surgical treatment options are below-the-knee amputation or joint fusion, which result in loss of motion of the ankle and can have poor outcomes.”
While patients who receive the Patient Specific Talus Spacer implant may still need to have fusion surgery or other procedures in the future, the goal of the implant is to preserve joint flexibility and motion.
“[This] action provides patients with a treatment option that could potentially reduce pain, retain range of motion of their joint and improve quality of life,” said Capt. Raquel Peat, Ph.D., Director of FDA’s Center for Devices and Radiological Health’s Office of Orthopedic Devices.
Additive Orthopaedics received the Humanitarian Use Device designation based on positive data from 31 patients who had a total of 32 talus replacement surgeries. Three years post-operation, the patients had decreased pain and better average range of motion in the joint. Of this group, only three had another surgery.
In addition to the Patient Specific Talus Spacer and other implants, Additive Orthopaedics is developing a suite of 3D-printed products for the entire foot, including bone segments and lattice plates.
“The Patient Specific Talus Spacer is another example of how 3D-printed devices can improve the standard of care. This is a tremendous regulatory win which took significant effort from our team,” Kowalczyk said.
Annie Zaleski is an ORTHOWORLD Contributor.




