
Recent advancements in the sports medicine world have centered on ACL repair, with new innovations aiming to significantly improve the long-standing techniques that have often left patients with less-than-ideal outcomes. Specifically, a new focus is gaining momentum to maintain native tissue and promote healing.
“Traditional treatment for most ACL tears involves a surgical reconstruction technique, in which graft tissue from the patient’s body or donor tissue is used to recreate the damaged ligament,” said Justin Boyle, Senior Product Manager, Knee Arthroscopy for Arthrex. “Over the past decade, there has been a renewed interest in primary repair as a potential treatment for certain patterns of ACL rupture.”
Scientific understanding has evolved over the last five to 10 years in tissue science and the ability to understand how to build structures, combine materials and generate data that demonstrates efficacy. Sports medicine is transitioning from adding mechanical strength or mechanical augmentation of a soft tissue repair to focusing on the role of biology and how the combination of strength and biology results in really differentiable long-term solutions.
There have been several promising approaches to the issue. With a large patient base of about 400,000 annual injuries in the U.S., ACL tears have been a pervasive problem that is only growing as the population ages and continues an active lifestyle. Sports medicine companies are seeking ways to stimulate biology and eliminate second-site harvesting and comorbidities and extend the healing for patients.
“The allograft argument continues to dwindle, and we really see the future in ACL as engineered products that stimulate healing, but also bring high strength,” said Jeff Conroy, CEO and Co-founder of Embody. “We really see the need as bringing an off-the-shelf, high-strength, load-bearing, biomimetic technology that is focused on repair and reconstruction not only in ACL, but in technology that extends to foot and ankle, medial and lateral ankle stability, and rotator cuff repair.”
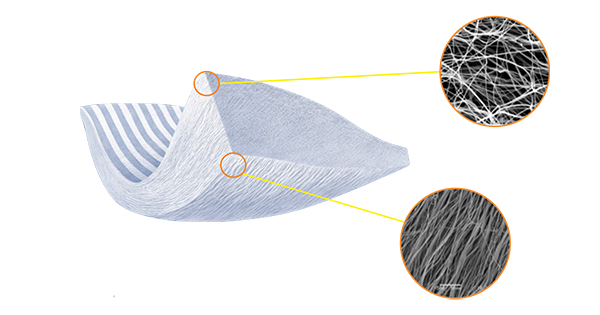

Harvesting the Power of Collagen
Embody has catapulted onto the regenerative sports medicine scene in recent years with their novel, collagen-based biofabrication techniques. In 2020, they launched their flagship product, TAPESTRY, an implant with a bioengineered micro-architecture and bio-stimulative, highly organized type I collagen chemistry specifically designed for tendon repair.
Their approach has been to have product developers work hand-in-hand with clinicians and key opinion leaders to understand the procedures and nuances of instrumenting the procedures, as well as aspects such as how time and cost come into play.
Knowing that patients have a range of injuries, where the majority require a reconstruction approach and a minority need repair, Embody intends to build tools that have the right characteristics for both reconstruction and repair with a long-term portfolio approach.
“Creating the opportunity for biology to make an impact is really critical,” Conroy said. “We’ve long had this problem of lack of innovation in soft tissue repair and augmentation. We’ve really focused on building a toolkit of technologies and particularly leveraging collagen science in a way to build an extensible platform so that we have a broad number of indications. We’re able to harvest the power of collagen in some cases where high strength is needed, such as ACL, and in some cases we stimulate a new tissue formation.”
As part of this approach to building a complete toolkit, Embody advanced their MICROBRAID and MICROBRACE collagen-based, high-strength suture products in 2020. They plain to launch these in 2022.
“Our vision is, the suture is the implant of the future,” Conroy said. “And when the implant is a woven or braided suture tape, or it’s a tape that plays both a load-bearing and a pro-healing or a collagen enhanced, biologic stimulating role, that is exciting because it means that we’re building technology that extends across some very important areas of which ACL is core in central.”


The SwiveLock® ACL Repair Kit is designed as a less-invasive treatment method for certain types of ACL tears.
A Comprehensive Solution
Sports medicine giant Arthrex has been involved in developing the ACL primary repair procedure since 1983. The company recently launched the SwiveLock ACL Repair Kit as a comprehensive implant system designed to repair and internally brace the ACL.
ACL primary repair with the SwiveLock ACL Repair Kit involves reattaching a torn ACL with SwiveLock anchors and high-strength FiberWire and TigerWire sutures. The repair uses an InternalBrace ligament augmentation, which acts like a seatbelt to protect the repaired ligament while it heals. The convenient kit contains all the surgical products needed for the ACL repair technique.
“These advancements may allow for a quicker return to activity and diminish pain following the procedure,” Boyle said. “While primary ‘repair’ will not replace reconstruction for all cases, the procedure, utilizing the SwiveLock ACL Repair Kit, is a valuable tool to help surgeons treat their patients better.”
The kit is indicated for use in proximal ACL tears, which results in approximately 16% of all ACL tears.1 In biomechanical testing, this repair technique has been shown to provide comparable knee stability to ACL reconstruction techniques with bone-patellar tendon-bone (BTB) autografts.
The first ACL primary repair with the new kit was completed on November 23, 2020, by Gregory DiFelice, M.D., an orthopedic surgeon at Hospital for Special Surgery in New York who pioneered this technique and developed the kit in partnership with Arthrex.
“The early feedback from this new implant system has been very positive,” Boyle said. “We are excited to offer our surgeons another innovative solution to help them treat their patients better. As a former collegiate athlete and former ACL reconstruction patient, it’s very inspiring to know there are now FDA-cleared, more minimally invasive treatment options available to treat ACL injuries. ACL repair certainly won’t replace reconstruction, but it is now proven to be a viable option for the right patient with the right tear pattern.”


Miach’s BEAR activates the body’s own healing to repair ACL tears.
Starting at the Source
Miach Orthopaedics has flipped the traditional ACL approach on its head—rather than looking at bringing in new tissue to repair the old, they have developed a technology to activate the body’s natural healing process from the inside out. Research has shown the ACL to be in a hostile environment where blood clots cannot form because of the type of fluid in the knee joint. When a blood clot cannot form, new collagen cannot form and the ligament cannot heal.
Miach’s Bridge-Enhanced ACL Repair (BEAR) implant enables the blood clot that is necessary for natural healing. Bovine tissue is highly cleaned, purified and shaped into a cylindrical implant about 45 millimeters long and an inch wide. It is very porous, allowing for the cells to move into the implant and absorb the proteins in the implant. A blood clot is then able to form and growth factors are released to start the process of healing.
“We create a favorable healing environment, and then the body takes over and it heals,” said Martha Shadan, President and CEO of Miach Orthopaedics. “So we’re not necessarily healing the ligament; we’re allowing the body to do its job.”
BEAR is injected with the patient’s blood during surgery. It is inserted between the torn ends of the ACL and is stabilized thereby suturing it to the two bones on either side of the kneecap, the femur and the tibia. It’s not sutured to the torn ACL, but rather nestled in next to the ligament. The torn ACL remains in place, retaining the natural insertion point of the native ACL. As the two ends come together and heal, the insertion points have not been disrupted and the ACL is restored back to its natural anatomy and position within the knee.
“As the new collagen starts growing, the implant itself is reabsorbed and it goes away within eight weeks,” Shadan said. “So it’s not permanent. It’s there to do a job and then it goes away, so you’re not left with things you don’t want in your body.”
Beyond the biologic issues, the challenge was to develop an implant that was straightforward, repeatable, and effective in stabilizing the knee while new collagen tissue was produced. Furthermore, promising preclinical and animal studies showed that patients who had BEAR implanted had no osteoarthritis following procedures, whereas some of those who were not treated with BEAR did.
Miach was granted FDA De Novo approval in December 2020. Currently, Miach Orthopaedics is monitoring the ongoing situation with the COVID-19 pandemic to determine the best time to launch. They plan on a limited market release in 2021 with orthopedic surgeons who have a high volume of ACL procedures and those who are at the forefront of innovative technologies.
Looking Ahead
As technology advances in ACL and soft tissue repair areas, sports medicine companies are looking at ways to simplify procedures through minimally invasive techniques and technology and construct materials and solutions that either mimic or stimulate native biology that are practical for surgical applications.
While Arthrex, Embody and Miach have new technologies on the market, other companies are developing products too. For example, Biorez is developing the BioBrace, intended to augment and reinforce a range of tendon and ligament procedures like ACL reconstruction.
Positive long-term patient outcomes are dependent upon innovations that go beyond the current standard of care.
“If we can significantly reduce or eliminate autografts by having off-the-shelf, high-strength, load-bearing, biomimetic and bio-stimulative products, and if you’ve got products that gradually remodel over time and are replaced with the patient’s own tissue with less surgical time, no second surgical site comorbidities, you’ve got innovation in ACL repair,” Conroy said. “My expectation over the next five years is that we’re moving to off-the-shelf solutions that address the complete range of benefits needed by patients.”
1. Clinical outcomes of arthroscopic primary anterior cruciate ligament repair: a systematic review from the scientific anterior cruciate ligament network international study group. Arthroscopy. 2020;36(2):594-612. doi:10.1016/j.arthro.2019.09.021
HT
Heather Tunstall is a BONEZONE Contributor.




