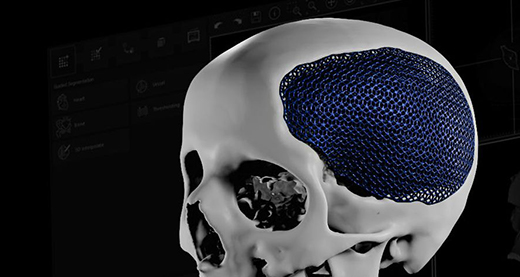
Materialise obtained CE Mark approval for most of its personalized orthopedic and craniomaxillofacial offerings, including 3D-printed anatomical models and patient-matched surgical guides and implants. Materialise is the first to acquire this kind of certification for a large, personalized, 3D-printed medical device portfolio. The company’s knee guides have brought procedural personalization into the O.R.
“The advantage of using personalized devices is that they add more predictability into the surgical setting and allow the treatment of any clinical condition, including the most challenging ones. Now having this CE marking for our larger portfolio will help bring these personalized devices to even more patients, removing some of the administrative constraints for our customers,” said Brigitte de Vet, Vice President and Managing Director of Materialise Medical.
Since its foundation in 1990, personalized solutions have been Materialise Medical’s core business. Personalization starts with planning, and the launch of Mimics software in 1992 enabled accurate, 3D image-based engineering, forming the base of advanced digital planning solutions available today. Materialise was also the first company to introduce personalized 3D-printed solutions into the operation room with the surgical knee guides’ launch in 2007. The CMF personalized implant launch in 2009 introduced a breakthrough in design with a porous structure in titanium that allowed the reconstruction of missing bone segments.




