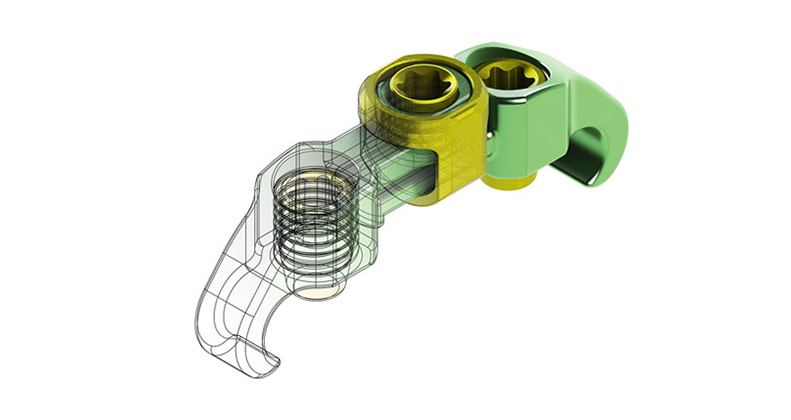
In this competitive environment where speed to market is vital, the design and prototyping phase of any project carries more importance than ever before. Companies that can collaboratively work with suppliers from the onset and communicate throughout the project will have a better chance of being successful.
We spoke with five suppliers with expertise in design and development to get their perspective on design and prototyping in the orthopedic industry:
- Phil Allen, Vice President of Sales and Marketing, Lowell
- John Ruggieri, Vice President, Engineering and Business Development, ARCH Medical Solutions
- Jennifer Palinchik, President, JALEX Medical
- Denis Leissing, Chief Executive Officer, Mediliant
- Ian Trotman, Engineer Director, Phillips Precision Medicraft
How has your design and development work with device companies evolved over the years?
Allen: One of the biggest evolutions in design and development work has been the amount of time invested before a product is manufactured. We now spend more time with our customers discussing design intent, manufacturability, inspection data requirements and a range of other factors before any metal is cut. When we spend the time upfront figuring out what dimensions and features are critical to performance and what’s needed during inspection, the process is much more efficient. It helps cut down on design iterations that can delay timelines.
We’ve seen a tremendous change in our development process since the outbreak of COVID-19. We are very fortunate to have a healthy, dedicated and safe workforce; our critical suppliers all across the country are doing amazing things, staying open, protecting their people. We haven’t pushed out a single delivery. We’ve also been communicating regularly with our OEM partners on deliveries, staffing and key supplier status. Technology has been a huge help in collaboration during our PPM meetings, design reviews and inspection planning meetings.
Ruggieri: A decade or more ago, a manufacturer’s participation was packed in at the very end of the product commercialization cycle. This often resulted in many design “tweaks” to allow for manufacturability. The change process back then was more tolerant of these adjustments. The risk aversion for such changes by the OEM was a bit more relaxed. I recall the size of the teams that were collaborating as being much smaller than they are today. The amount of effort and resources needed to participate, migrate and manage through a development project is exponentially larger now. It takes a big commitment on the part of a contract manufacturer to have these skilled and experienced teams available to take on these projects. There is also a significant commitment from the OEMs to keep these teams in constant use, thereby warranting their existence and availability for their next project.
Palinchik: Having a more collaborative experience with our clients has helped move the design process needle for us at JALEX. Improved virtual tools for communicating and the ability to create designs and show them quickly has certainly streamlined and expedited the process. There are so many elements required from a product development standpoint, and they all take an extensive amount of time to complete. Being able to push through each phase of the lifecycle relatively quickly allows us to achieve market clearance in a more expeditious manner.
Leissing: The requirements from the customers are becoming more standardized. It’s more process-oriented now, which, for us, is a useful way to make sure that we don’t forget anything – that we get all the inputs from the customers for any development work, and also make sure that we can evaluate the project correctly from the beginning. After that, the development process is more of a device-type approach with a stage-gate process and customer milestone or internal milestone. CAD/CAM systems are the standard now, and I would say that 95% of the customers come to us with models, which wasn’t the case before. That helps to save time. Also, the more we’ve got experience with applied process type, then the quicker we get, especially with tooling. We are also using more 3D printing to help us in the pipeline phase, especially.
Trotman: Our creativity and ability to develop designs that support our customers’ brands and provide ease of use during surgical procedures have evolved and improved over the years. However, it’s the increase of regulatory requirements that continues to challenge our normal design-to-production timeline for both ourselves and the OEMs that we serve.
What trends do you see with design and prototyping? Upon what are they based?
Allen: Making devices smaller and more complex is one of the ways that companies are innovating and trying to deliver on better outcomes. When a part becomes more complex, it’s really easy for a design to become more complex as well. We’ve been working with our OEM partners on using standards like geometric dimensioning and tolerancing (GD&T) to make their drawings and design intent easier to understand, which can help improve manufacturing, inspection and validation.
When we’re talking to a customer about their design intent, rapid-prototype samples (both machined and 3D printed) have become indispensable. When we make a sample of a part, we can more easily confirm and ask questions about how parts should fit together, what a finish should look like, and many other criteria important to success. By figuring out these details before manufacturing, we can make product development quicker and smoother for everyone involved.
Ruggieri: There is more pressure on getting the details right with only one pass through the design cycle. Rapid prototyping is a must. Deep and meaningful engagement between designer and manufacturer is required at a very early stage, and a high level of trust, confidence and collaboration is mandatory between the organizations. Information needs to flow freely and constantly without waiting for a fixed meeting schedule to address open issues.
The most significant factor I have seen contribute to the success (or not) is having the prototype team and organization remain the same as the project moves from prototype to full production. There’s just not enough time to introduce new players at either of those phases, and there is too much collaborative knowledge at stake that may otherwise miss the transfer from one phase to the next. The most successful contract manufacturer and OEM teams build their enterprises to be good at both phases with a seamless flow from one to the next.
Palinchik: There are so many more options than before to create quick turnaround prototypes that are essential during the initial stages of product development. This is due in part to the increasing evolution of 3D printing and traditional manufacturing companies that have created processes to follow this quick turnaround need, as well. Being able to create a design and get it in the hands of our clients as soon as possible drives innovation and client engagement. It allows for better collaboration between designer and inventor, which is important for bringing novel products to the market.
Leissing: Companies are more open to discuss the design specification before the design freeze, which is good. We see more partnerships in the design phase. We also see more justifications requirements, especially during the validation phase, which is linked to the regulatory requirements. Sometimes, we have customers coming to us to develop a second source or product. It’s more difficult to request a design change when the product is already CE Marked, because the burden is increasing for a design change on the OEM side with their notified body. If we want and if we need changes, we need them before the design freeze.
Trotman: We’re seeing our customers move away from hybrid designs such as cases/trays made with a combination of plastic and sheet metal as well as vacuum formed designs. The sterilization efficacy of sheet metal is far greater than plastic because it dries faster and leaves no water spots or patches for bacteria to form upon. The mold costs associated with plastic and vacuum formed designs, which increase if changes are made to a product line. We’re also seeing customers use the prototyping phase of a project to address not only the form, fit and function of a design, but also to understand the impact of regulatory requirements and how to best address or mitigate potential issues.
What advice would you give device companies on ways to shorten the time and decrease cost during the design and development phase?
Allen: Aligning your team with your manufacturer’s is one of the most important ways to improve any device’s development. We do this with thorough pre-production meetings when our team talks with our customer’s inspection, engineering, operations and purchasing reps about all details of a product’s performance and manufacture. By spending time discussing design intent and reviewing drawings and models, we can better understand how a device should work and use these to improve the manufacturing process.
It’s also important to talk about inspection and validation requirements. For example, if we need to do any destructive testing for validation, how do we account for those parts? What data do we need to collect to meet your inspection requirements? What equipment will be used to check the part? Taking these elements into account can help avoid unexpected surprises, which can lead to timeline delays.
Ruggieri: My advice would be to find a partner you trust who has the mindset of having the patient’s best interest as their first priority. Engage early, engage often and make sure there are plenty of interactions on location and in person.
Palinchik: Understand the big picture first, then develop an appropriate plan to execute. This should entail market needs, user requirements and commercialization, among other things. Also, one of the biggest misconceptions that many device companies or inventors have is how critical it is to understand the regulatory landscape of the product you are trying to create. This should always be the very first item to recognize before embarking on the design and development activities themselves. The regulatory pathway dictates every stage of development, and having a robust strategy upfront will help reduce time and cost in the long run. It also provides a checklist of what the design must go through to prove safety and effectiveness.
Leissing: Start as early as possible with their contract manufacturer and don’t wait. Even if it’s only a draft and not yet a design per se, sometimes the contract manufacturer can help with the technical solutions on the design because contract manufacturers see a lot of different devices and they know what’s working and what doesn’t work. Align the validation strategy very early, because we see that validation is becoming a heavier load of the work and it could have a significant implication on the customer’s time.
Trotman: The number one piece of advice we would offer is implementing a design freeze. A design freeze is the point in product development where the design is considered “released or complete.” Sometimes we’re required to work on projects where the instrument design is not fully defined and changes can occur while cases are mid-design, this makes our job more challenging, time-consuming, and often adds to the final design costs. It will save time and money if instrument designs are fully defined before we start a project.
Photo courtesy of Lowell
HT
Heather Tunstall is a BONEZONE Contributor.




