Use of titanium, specifically the core specialty alloy titanium 6-aluminum 4-vanadium (Ti64), continues to grow because of its premium performance and proven biocompatibility. An increasing number of orthopedic companies are utilizing additive manufacturing with titanium powder to drive growth, highlighted by the increased use of biologically compatible titanium for orthopedic spine, knee and hip implants. Yet this rapid growth in demand can strain supply chains, leading companies to look for new, proven solutions.
Titanium Powder for Additive Manufacturing
Different additive manufacturing technologies utilize varying size distributions of powder, and not all powder atomization methods are suitable for titanium alloys, due to the deleterious effect of refractory inclusions on fatigue and toughness. This leaves crucible-free plasma atomization (PA) and Electrode Inert Gas Atomization (EIGA) as the preferred methods of producing powder for additive manufacturing. PA uses a pre-alloyed wire fed into plasma torches, creating molten droplets that rapidly solidify as highly spherical powder particles. EIGA continuously feeds a rotating, high-purity, pre-alloyed bar into an induction coil to form a molten stream that free-falls directly into a high-velocity inert gas, producing highly spherical powder particles. The EIGA method has multiple advantages over the PA process, including the use of bar feedstock as opposed to wire, allowing for greater supply chain freedom and flexibility for tailored compositions.
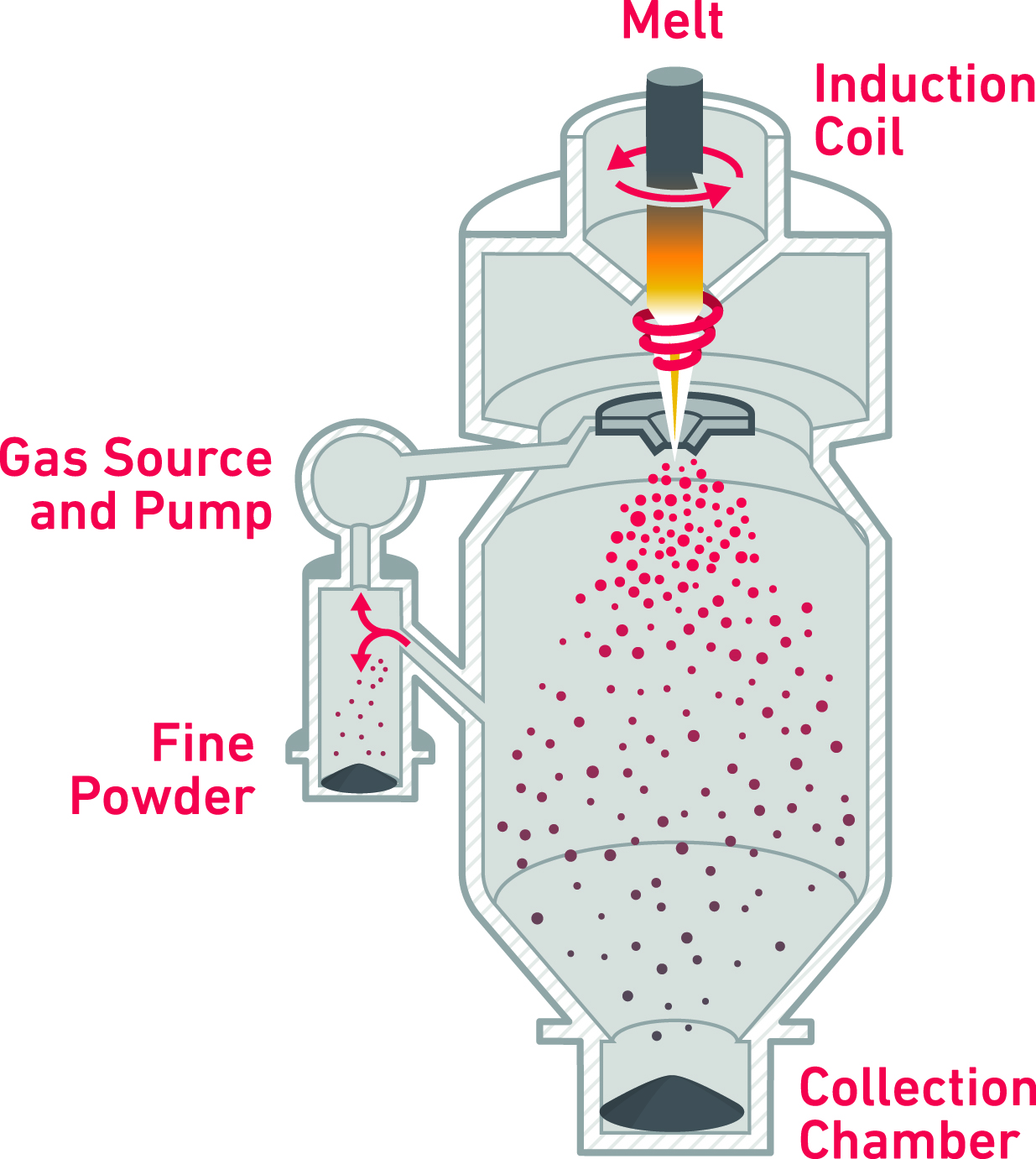
Electrode Inert Gas Atomization Process
Minimized Oxygen Content
Oxygen, nitrogen and hydrogen are interstitial elements within the Ti64 alloy that have a strong influence on the mechanical properties of the end part, and improper control can result in parts with insufficient properties. Oxygen content is also particularly important for additive manufacturing production strategies designed for high material re-use. Many additive facilities blend powder or use “topping up” strategies, where powder picks up oxygen during the additive manufacturing lifecycle. Understanding the effects of these powder strategies and minimizing oxygen content during atomization are essential to successful production with additive manufacturing.
Powder manufactured by EIGA gains as little as 100 ppm of oxygen from the feedstock, and the bulk oxygen content of bar feedstock is typically lower than that of wire. This results in oxygen content in EIGA powder between 200 to 300 ppm lower than that of PA for equivalent alloys.
Equivalency Study
Carpenter Additive carried out a powder equivalency study to compare the properties of Ti64 ELI powder produced by the EIGA and PA processes. The tests in the study evaluated several qualitative and quantitative measures aligned with the requirements of medical device OEMs. The results showed the following:
- Oxygen level – The EIGA supply chain includes less feedstock processing, resulting in lower oxygen levels.
- Contamination – The absence of any refractory components in the EIGA process minimizes the risk of high-density contaminants, such as tungsten.
- Density – The particle skeletal density of the EIGA and PA samples are equivalent, indicating no difference in internal porosity.
- Flowability – The quantitative flow measurements used in this study indicate that the EIGA sample has superior flow properties despite having a finer particle size distribution.
- Morphology – The sphericity and agglomeration of EIGA and PA powders is equivalent, as examined through quantitative and qualitative techniques.
The results showed that the sample produced by EIGA proved superior in terms of chemistry and flowability and exhibited no statistically significant differences in morphology or density. The full results of the study can be downloaded as a white paper or watched through an on-demand webinar. In the coming months, Carpenter Additive will expand the results of this powder study to investigate the equivalency of final-end use parts produced with both EIGA and PA Ti64 powder.
Dr. Gaurav Lalwani is the Global Medical Applications Engineering lead at Carpenter Technology Corporation, where he manages the technology portfolio and new product development strategy for the medical business unit. He is currently engaged in the development of novel wrought and additive solutions for the medical device industry. With a MS and Ph.D. in Biomedical Engineering from Stony Brook University with a focus on materials science, tissue engineering and regenerative medicine, Dr. Lalwani was the co-founder of Millennial Material & Devices and Vice President of Research for Theragnostic Technologies Inc.— seed stage biomedical startups. He is an active member of ASTM, MRS, BMES, ECS and other societies and holds a keen interest in promoting inclusive education and food safety by supporting and advising organizations focused on social entrepreneurship.