Embody closed a $9.3 million Series A funding round. Proceeds will support new hires and the commercial 2H20 launch of the Tapestry® collagen-based microfiber implant for Achilles tendon and rotator cuff repairs.
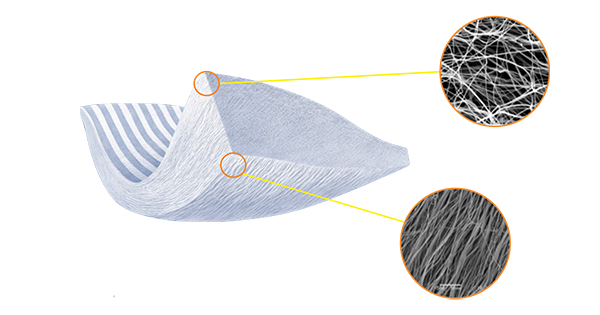
Tapestry’s design is based on research of collagen increasing the activity of recellularization by growing new aligned tissue over surgical repairs. The collagen-based nanofiber graft, formed with electrospinning and microfluidic fabrication techniques, is resorbable and designed to mimic the architecture of the native tendon.
Embody’s novel biomaterial for soft tissue repair will have an initial focus on orthopedic applications including Achilles, rotator cuff and ACL repair. Unlike conventional acellular dermis or extracellular matrix materials, Embody’s proprietary process yields an engineered biointegrative collagen-based construct designed to promote new, host-generated, dense, regularly oriented tendon-like tissue.
“This financing represents a significant step in the growth of Embody,” said Jeff Conroy, Embody’s Co-founder and Chief Executive Officer. “We are now in a position to execute on our goals of commercializing Tapestry, our first in a series of soft tissue repair products for Achilles, rotator cuff and knee ligament injuries.”
Conroy believes that the Embody approach can generate similar or superior results to Smith+Nephew’s REGENETEN with some other advantages, including on the economic side, making them a strong competitor in the rotator cuff space.
“Our novel additive manufacturing process (BioSpin™ hybrid electrospinning/pneumatospinning) combines clinical-grade collagen and Poly (D, L-Lactide) (PDLLA) to produce a controlled, highly aligned, cell-infiltration-friendly microstructure with controlled degradation profile, tailored fiber diameter, specific pore size and mechanical properties that mimic native tissue,” he said. “The materials are engineered based on the clinical application and the devices are carefully tailored to address clinical needs for each indication.”
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




