While the orthopedic device industry has changed its tune on metals vs. polymers over the last 20 years, the push for material innovation has remained. Entering a new decade, orthopedics is experiencing another wave of polymer innovation that provides greater design freedom and compatibility with the body at a lower price tag, according to industry experts. While trends can differ by application, the overarching characteristics that emerge are polymers that are more porous, flexible, strong, resorbable, biocompatible, safe and cost-effective than the last generation.
As a long-time provider of polymer materials to medical device companies of all sizes, Evonik sees changing demands firsthand.
“Medical device companies are looking for safe, effective and versatile materials with proven benefits that help enhance device performance and improve patient outcomes,” said Balaji Prabhu, Director, Medical Device Competence Center, at Evonik. “In addition to attaining property outcomes to improve flexibility, strength, behavior or functionality, it is important that the materials can deliver high levels of purity, traceability and supply security.”
In response to these evolving needs, Evonik has taken steps such as expanding its portfolio of bioresorbable RESOMER polymers to meet demands for customizable mechanical properties and application versatility, as well as adding a surface modification technology, Endexo, to enhance the biocompatibility of medical devices that come in contact with blood, tissue or other biological fluids.
A great deal of the polymer innovation, though, has been driven by startup device manufacturers.
“When you think of the well-established companies that are successful in the marketplace, [those companies] have deep expertise in a couple of materials,” said Mira Sahney, President and Chief Executive Officer at Hyalex Orthopaedics. “Once you step outside of that, they’re less well-suited in their infrastructure and scope to address some of the new polymers.”
We spoke with five orthopedic startups about the properties and applications of their polymer innovations, as well as their commercialization paths.


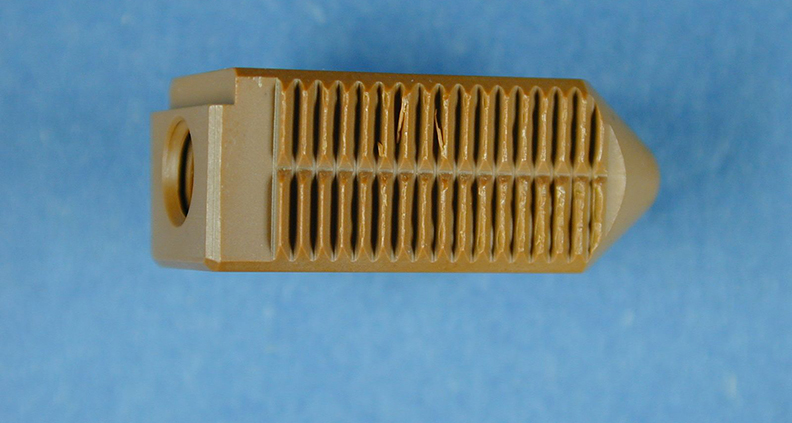
HYALEX synthetic cartilage maintains a low wear profile and high lubricity, even at high loads.
Cartilage
Articular cartilage is critical for orthopedic health, providing a lubricated surface for joint articulation and load transmission. However, cartilage is devoid of components that allow intrinsic healing and repair, so it continues to degrade over time until, for some, total joint replacement is inevitable.
“When we look at trends, we have an aging population and people are generally both heavier and more active, therefore, putting more load on that cartilage,” said Sahney. “Cartilage has become the Holy Grail of orthopedics. Instead of going to total joint replacement, is there a way to just address the cartilage issue?”
Cartilage is a bearing material, rather than a fixation material, in that it rubs against itself when people walk and move. A suitable synthetic material alternative to cartilage would need to have low wear and high strength over a long period, while providing lubricity for movement and biocompatibility to comply with natural materials in the body.
Cobalt chrome and polyethylene are two bearing materials that are found in traditional orthopedic implants, but which have limited compatibility against articulating cartilage. Hyalex’s synthetic cartilage polymer material mimics the form and function of the native hyaline cartilage found in many joints. It provides low wear and seeks to retain the joint’s natural anatomy, replacing only the lesions in the diseased portion of the joint. The company has also developed a cement to adhere Hyalex synthetic cartilage to the bone.
Sahney said that the synthetic cartilage can be used in any diarthrodial joint. For example, it could be used to replace half a joint and bear against natural cartilage such as in a hip hemiarthroplasty, as well as when replacing a piece of cartilage to correct a lesion or defect in a knee or ankle.
“If you have healthy bone, we’re trying to help avoid a total joint replacement while being cost-effective and minimally invasive,” she said.
The company is in the preclinical phase of development, proceeding with standard tests for new materials before pursuing clinical studies.
Meanwhile, BioPoly has offered a cartilage polymer since 2012. Its President and Chief Executive Officer, Herb Schwartz, Ph.D., said that the company has more than eight years of positive clinical results in the knee using materials with decades of clinical experience. BioPoly is made from hyaluronic acid and an ultra-high-molecular-weight polyethylene (UHMWPE) that has been used for decades in total joint arthroplasty. Hyaluronic acid helps provide a nearly frictionless surface in a synovial joint, while UHMWPE allows the implant to carry significant anatomical loads. This makes it applicable for focal cartilage repair and replacement as well as joint bearings and liners.
“The end result is a robust, long-lasting orthopedic material that functions as cartilage, keeping the patient pain free,” Dr. Schwartz said. Some may wonder whether cartilage polymers could ever eliminate the need for total joint replacement altogether, but Dr. Schwartz doesn’t think so.
“Some surgeons who implant BioPoly have commented that the osteoarthritis in their patients has not progressed over five years or more,” he said. “Some patients with focal cartilage defects just have subpar cartilage; therefore, no matter what we do, their joints will likely continue to deteriorate. However, if we can give them some more active years without pain, then I think we have done the right thing for them.”
Dr. Schwartz sees reducing wear rate and extending total joint life with a robust polymer as the main interest for the industry.
Still another solution, Ortho Regenerative Technologies’ Ortho-R biopolymer molecules, can be mixed with platelet-rich plasma to deliver biologics to increase healing in injured tendons, meniscus, ligaments and cartilage. Rather than using hyaluronic acid or collagen as a natural biopolymer, the product uses chitin extracted from shrimp shells. The company’s President and Chief Executive Officer, Claude LeDuc, explained that chitin is the second most abundant matter after cellulose. He describes it as a safe, non-toxic, natural material that is biodegradable, fungistatic and bactericidal with hemostatic properties.
During the manufacturing process, chitin is transformed into chitosan, a deacetylated form of the original molecule, with stringent manufacturing norms to meet FDA and other regulatory compliances.
“Ortho-R chitosan natural biopolymer molecules are positively charged and therefore muco-adhesive (sticky) to the negatively-charged soft tissues of the human body,” he said. “It is, therefore, a perfect delivery system for all orthobiologic musculoskeletal applications.”
The company is also exploring an osteoarthritis combination product, as well as other orthopedic applications such as bone void filling and bone fusion.
Ortho-R is in the final preclinical stage of a rotator cuff tear repair study in sheep while analyzing MRI data and histology samples from 2019. The company looks forward to moving into the clinical stage and performing surgeries at U.S. shoulder centers in 2020.


M.M.A. Femoral Ball and Acetabular Liner
Joint Replacement
Progressive polymers are at work when a joint replacement is necessary, too. One focus of these materials has been solutions for patients with metal allergies or those who suffer from tissue inflammation or temperature-related pain due to metal.
Originally designed for jet bearings as a substitute for steel, the MP-1 polymer from M.M.A. Tech has found an application in hip and knee replacement and dental implants.
Alisa Buchman, the company’s Chief Technology Officer, said that the strong material offers a longer lifespan than metal and withstands dynamic motion in the body for more than 30 years. It’s also self-lubricating and becomes smoother the more that it moves to provide a better range of motion. The MP-1 polymer’s ability to withstand extremely high temperatures eliminates the chance of creep, fatigue or dimensional changes at body temperature. Excess particles generated by movement have been too large for macrophages to swallow, removing the chance for infection. Finally, MP-1 polymer is visible in x-ray scanning, which can enable a better follow-up.
Applications for MP-1 include hip liners, tibial components, intramedullary nails and screws and dental implants.
MP-1 is a pure polyimide polymer produced from four monomers to create a powder. This powder is compression molded into a block or to a near-net-shape product and machined into the final medical device. Buchman said that her company’s polymer has been used in more than 100 surgeries in Israel and New Zealand, with “100% clinical success.” The company is now in postmarketing commitments.
Additionally, in light of the new medical device regulation in Europe, M.M.A. Tech plans to become a supplier to device companies rather than an OEM serving hospitals.
“The new regulation will not allow selling implant parts, only the whole system,” Buchman said. “The hip system consists of four parts: stem, femoral ball, liner and cup. We can currently produce the liner, and possibly the femoral head, but not the stem and cup, which are metal. Therefore, we need to cooperate with orthopedic companies that are producing the other components.”


HAPPE Spine Cervical Interbody spacer implant formed entirely of HA-reinforced PEEK polymer.
Spine
Medical devices for the spine were primarily made of titanium until the advent of PEEK (polyetheretherketone, a thermoplastic polymer) in the late 1990s. Research shows that PEEK can provide more porosity for bony ingrowth, but isn’t as easily accepted by the body as was titanium. With 3D printing, device companies have the opportunity to make titanium porous, and the pendulum is starting to swing in the market again. Still, some companies continue to innovate in PEEK.
“When looking at the foundation of bony fusion, there are three criteria that need to be met. The medical device must be biomechanically stable, porous and bioaccepting,” said Doug Snell, Engineering and Program Director for HAPPE Spine. “Most devices in the market only meet one or two of those criteria.”
HAPPE’s dual density HA porous PEEK technology aims to meet all three. Its PEEK material is inherently strong and porous. Also, while natural PEEK use typically results in a biofilm that interferes with fusion, HAPPE adds hydroxyapatite, a calcium phosphate that helps increase the device’s bio-acceptance. Furthermore, the company uses hydroxyapatite in whisker form rather than as a particle for added strength.
“The product actually looks like bone,” said Dave Blue, Vice President, Sales and Marketing of Genesis Innovations Group, a HAPPE Spine investor. “The opportunities are really extensive anywhere you’re trying to do a bony fusion because we can design where we want strength and pores. It’s also cost-effective, which is important because in health care you literally can’t come out with anything innovative that’s much more expensive. You have to help drive down costs.”
HAPPE expects FDA 510(k) clearance by the end of 2020.
The Future of Polymers in Orthopedics
Evonik’s Prabhu sees personalized medicine as an emerging area of market focus. He expects the combination of 3D printing technology and versatile biomaterial platforms will address such patient-centric solutions. The company recently launched reportedly the first bioresorbable polymer in powder form suitable for the high-resolution 3D printing of medical devices.
“Instead of surgeons having to modify one-size-fits-all implants during the procedure, custom implants with precise geometries can now be rapidly created with the help of advanced 3D technologies,” he said. “This advancement can help to reduce surgery time, accelerate patient healing, eliminate the need for additional surgical procedures for implant removal and reduce the risk of metallic allergies.”
LeDuc of Ortho Regenerative Technologies said that research universities and corporate communities are aware of the needed evolution in polymers, and so he expects continued innovation.
“Like the discovery of petroleum more than a century ago, there will be thousands of new materials derived from naturally available materials or created using synthetic chemistry, constantly mimicking or improving the human body’s physiology to treat or cure diseases, heal trauma and regenerate new tissue for various applications,” he said. “The Holy Grail for any researcher is to invent something new and then have the industry take over to translate the invention into an affordable and thus highly used innovation.”
Kathie Zipp is an ORTHOWORLD Contributor.




