In the weeks following the annual NASS meeting, spine segment headlines kept rolling in. Zimmer Biomet introduced its first porous titanium implants to be additively manufactured. Aegis added expandable cages to its portfolio, and CarboFix launched its first Vertebral Body Replacement system. These new products extend important lines for each of these companies; therefore, we’ve recapped these late announcements below.
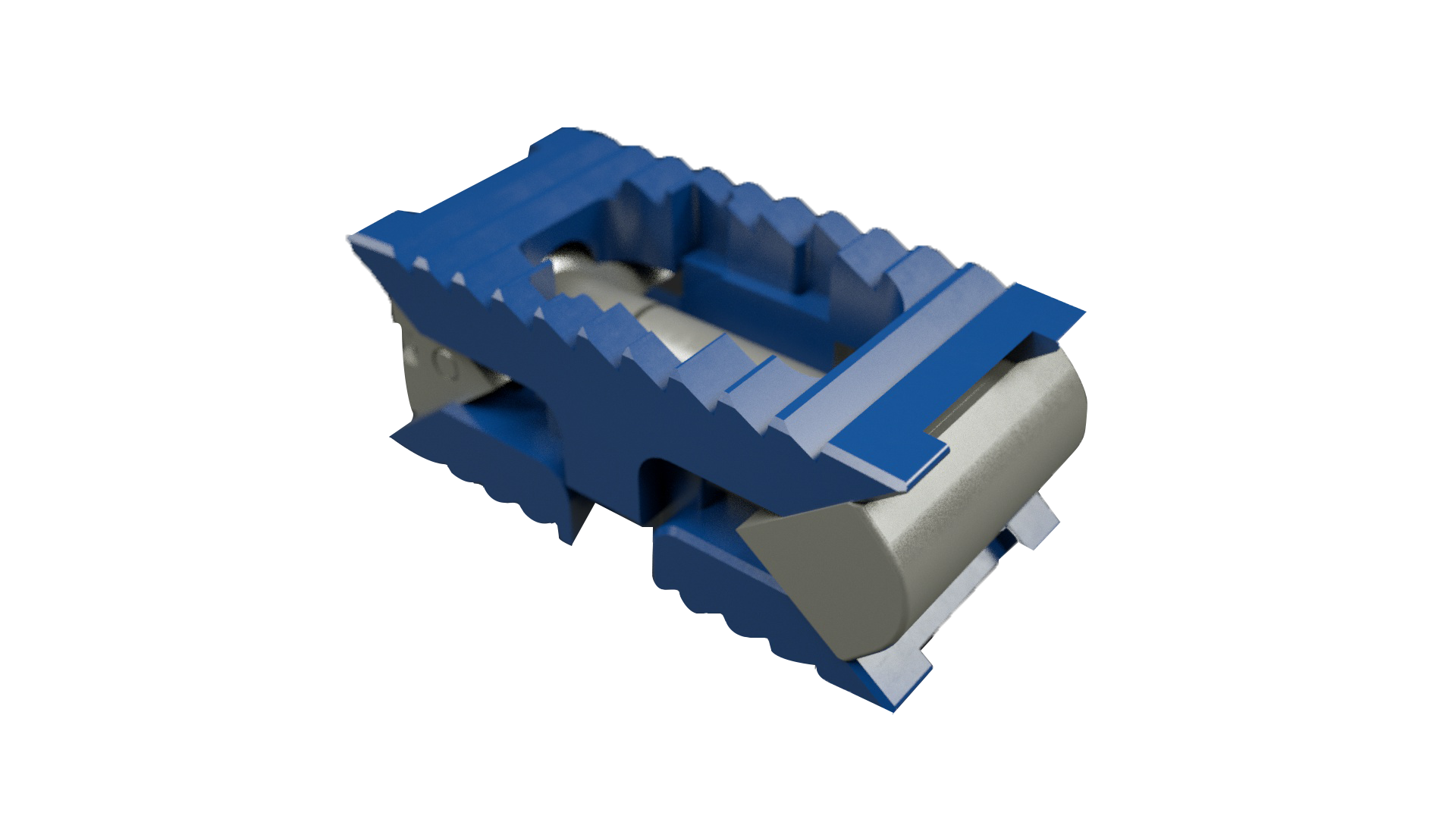
Zimmer Biomet launched the TrellOss-TC (pictured above, TLIF curved) Porous Titanium Interbody Platform. The 3D-printed titanium interbody features a scaffold structure with 70% porosity and a 7 micron roughened surface topography to support adhesion and bone ingrowth, and includes an integrated articulating mechanism which allows the implant to pivot in situ up to 55°.
TrellOss-TC, TrellOss-C (cervical) and TrellOss-TS (TLIF straight), the latter two of which were launched in August, comprise the company’s first porous titanium implant platform manufactured through a 3D printing/additive manufacturing process. The company plans to add lateral, anterior and stand-alone devices in 2020.
Aegis Spine, subsidiary of Korea-based L&K BioMed, commenced U.S. launch of AccelFix Expandable Cages following clearance of the devices in September.
The system comprises sterile-packaged titanium alloy-based TLIF/PLIF cages (AccelFix-XT, pictured right), an expandable lateral interbody (AccelFix-XL), and an Anterior-to-Psoas expandable interbody specifically contoured to avoid the contralateral nerve root (Accelfix-XTP).
AccelFix devices are complemented by a 5.5 rod spinal fixation system which earned FDA 510(k) clearance earlier this year.
CarboFix is launching the CarboClear Carbon Fiber Vertebral Body Replacement (VBR) system to replace collapsed, damaged or unstable vertebral bodies following tumor or trauma. CarboClear VBR joins the CarboClear Pedicle Screw and CarboClear Fenestrated Pedicle Screw.
The device is a carbon fiber-based implant with integrated porous Ti-alloy endplates. Potential advantages of carbon fiber devices include enhanced radiation therapy planning abilities and enhanced follow-up abilities due to artifacts-free imaging. Further, optimized fatigue strength supports patients with impaired healing processes. The VBR is intended for use with supplemental fixation and optional allograft or autograft.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




