Numerous spine companies focus on manipulating PEEK to promote osseointegration with interbody devices, but Vallum believes they are the only one addressing the seldom-discussed hydrophobic and bioinert drawbacks of the polymer.
Vallum has developed proprietary Accelerated Neutral Atom Beam (ANAB) processing technology, which creates a nanotextured surface that reportedly better fuses the bone and the implant and fosters bony growth. In 2018, Vallum received FDA 510(k) clearance for an interbody with its PEEKplus® nanotextured surface, created by the ANAB process.
The Water Factor
PEEK is a standard material used in interbody devices for spinal fusion. Among PEEK’s positive qualities is a strength comparable to steel. It is inert, radiolucent and biocompatible, and has a similar modulus to bone. But PEEK is not bioactive, because it is hydrophobic, which can lead to poor bony ingrowth.
Hydrophobic materials repel water, and since human bones are made up of nearly 31% water, a hydrophobic device will not promote osseointegration. Hydrophilic substances are more favorable to facilitating bone growth, as they interact better with water.
“The PEEKplus nanotexture surface that ANAB processing creates on the surface of PEEK is hydrophilic and bioactive,” said Stephen M. Blinn, President and Chief Executive Officer of Vallum. “Published in vitro and in vivo studies have shown statistically significant improvement in cell proliferation and osseointegration with a PEEKplus hydrophilic nanotextured surface compared to a conventional hydrophobic microtextured PEEK surface. Therefore, the purpose of superimposing a nanotextured surface onto the existing microtextured surface of a PEEK interbody device is to improve osteoblast function and osseointegration.”
Though there are commercially available PEEK interbody devices with coatings, porosity and chemical infusions intended to improve osseointegration, none of these devices address the underlying problem of PEEK being hydrophobic and bioinert, according to Blinn.
“Additionally, each of those approaches compromises one or more of PEEK’s positive qualities,” he said. “ANAB processing makes PEEK hydrophilic and osseointegrating without compromising any of its positive qualities.”
How Does ANAB Work?
PEEKPlus is not a coating. It is not porous PEEK. Chemicals are not infused into the PEEK. The ANAB process is applied to a completed device to create a nanotextured surface that makes PEEK osseointegrate in spinal fusion applications.
“The ANAB processor accelerates neutral argon atoms at high velocity and scans them across the surface of a fully-manufactured PEEK interbody device in the last step of the manufacturing process,” Blinn said. “Upon impact, each atom penetrates approximately 2-3nm into the surface of the PEEK, creating a picosecond thermal spike that turns the PEEK’s organic material at the site of impact into a gas, leaving a 20-50nm concavity in the surface of the PEEK.”
Blinn compares the concavities to dimples on a golf ball, but 4 million times smaller. The millions of impact points create a uniform nanotextured surface that is superimposed on the PEEK’s microtextured surface, leaving the bulk PEEK material unaltered.
Studies have shown that nanotexturing below 100 nanometers improves the osteoblast functions necessary to grow bone and to promote fusion. Vallum’s technology creates 20-50nm concavities, compared to titanium-coated and porous PEEK surfaces exhibiting 300,000nm holes.
In a Good Laboratory Practice sheep spine implant study conducted at AccelLAB in Canada, the quality of new bone formation at the cranial and caudal surfaces of implanted interbody devices scored higher for PEEKplus over titanium-coated, hydroxyapatite-infused and untreated PEEK. Further, PEEKplus was statistically significantly superior to titanium-coated PEEK (p<0.036) and regular PEEK (p<0.001).
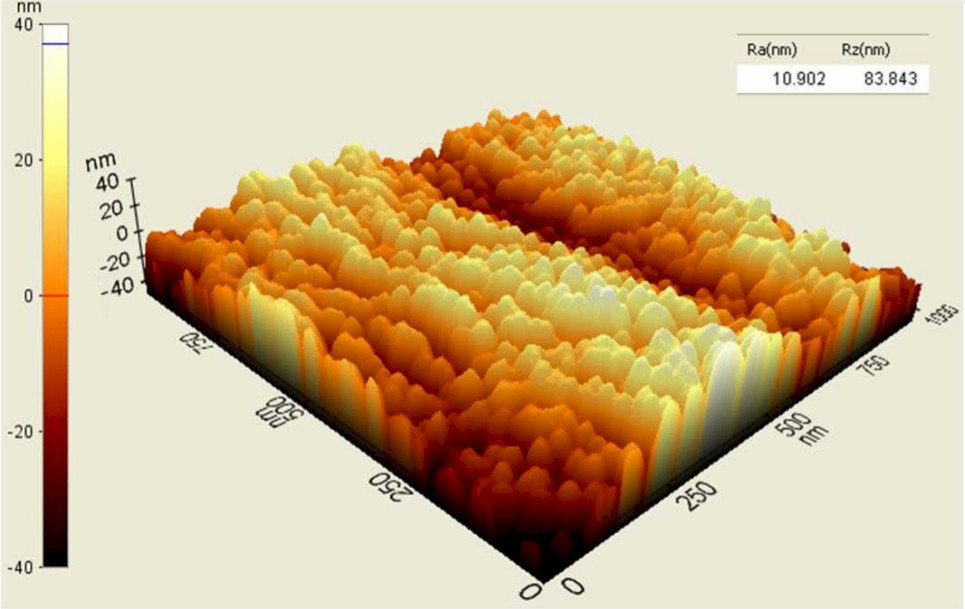

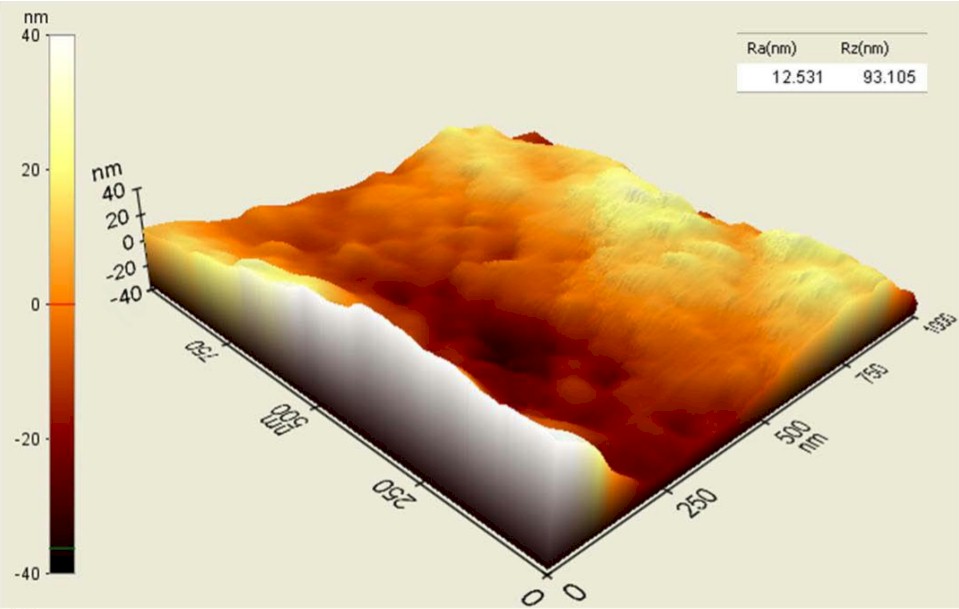

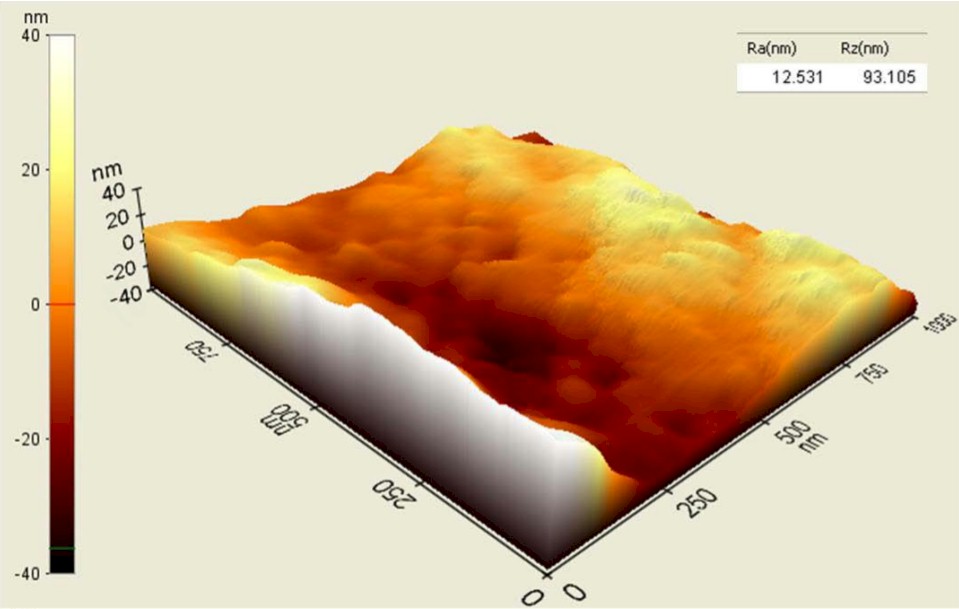
Processed PEEK (top) vs. Unprocessed PEEK (bottom)
Surface Texture Simplicity
Vallum’s ANAB nanotexturing technology can be applied to any fully-manufactured PEEK interbody device without altering its design or size, and without affecting mechanical or chemical properties, the company said.
To create the PEEKplus nanotexture, Vallum uses a proprietary, patented ANAB processor to dispel several quadrillion argon atoms/cm² across the surface of the device. The processor incorporates fully automated Rockwell Automation control software, has a high-volume throughput processing chamber, delivers precise device-to-device control of the nanosurface of each interbody device, and is operated in a certified class 7 clean room.
The ANAB processor and processing protocols for PEEKplus are fully developed, and PEEKplus nanotexturing is FDA-cleared as of July 2018. It is the first and only FDA-cleared nanotexturing on PEEK. Vallum is in discussions with leading device companies in an attempt to make PEEKplus the standard of care.
“We see no impediment to quick commercialization by a strategic partner, because any company’s current line of PEEK interbody devices can become hydrophilic, osseointegrating PEEKplus interbody devices with the addition of one simple ANAB processing step,” Blinn said. “We know of no other technology that can create a nanotextured surface like PEEKplus on PEEK.”
Images courtesy of Vallum Corporation.
HT
Heather Tunstall is a BONEZONE Contributor.




