Imagine a solution that enables regeneration of tendon-like Achilles tissue that can be measured in weeks—not months. What if the body could heal itself with assistance from biomaterials tailored to the individual, rather than relying on foreign substances or subpar cadaver grafts that may be rejected or cause further damage? Think of what it could be like for an athlete or soldier with a tendon tear to return to pre-injury readiness rather than having to decide on a new career.
Embody, Inc. seeks to make all of that a reality. The Virginia-based company is developing an approach to tendon and ligament repair that involves collagen-based implants for soft tissue restoration and regeneration.
A New Approach
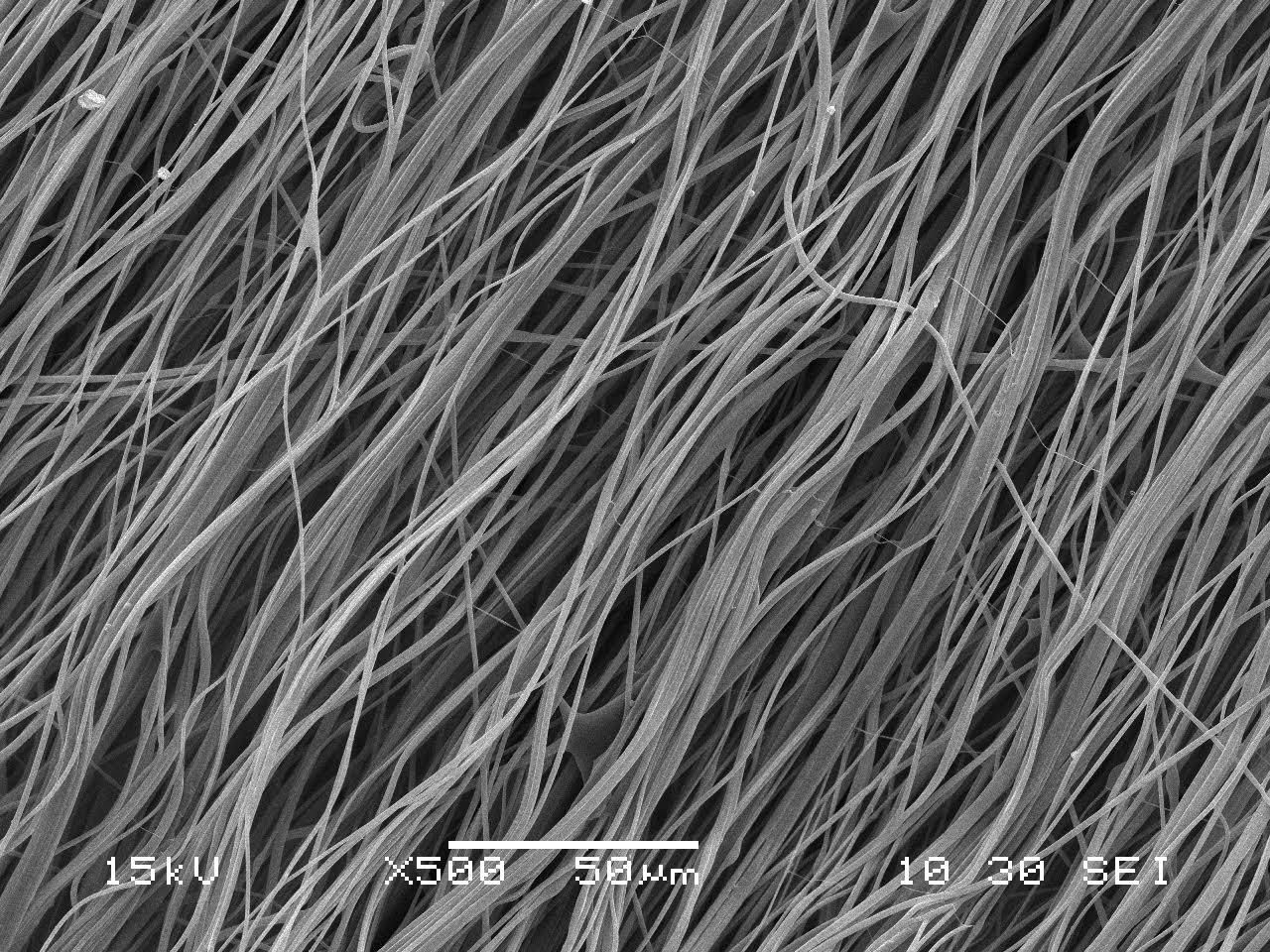
Embody’s novel biomaterial for soft tissue repair will have an initial focus on orthopedic applications including Achilles, rotator cuff and ACL repair. Unlike conventional acellular dermis or extracellular matrix (ECM) materials, Embody’s proprietary process yields an engineered biointegrative collagen-based construct designed to promote new, host-generated, dense, regularly oriented tendon-like tissue.
  | ||
| This scanning electron microscope image of the Embody Tapestry product shows a highly aligned microstructure as an analog to human tendon. |
“In many orthopedic applications like Achilles or rotator cuff repair, with traditional materials like allograft or acellular dermis, you are limited to the void fractions, porosity, strength and bioactivity of the raw material,” said Jeff Conroy, CEO of Embody. “However, we are able to tune the attributes to mimic the native tissue intended to enhance cell remodeling with greater cell adhesion (tenocytes) and more tendon-like tissue formation. This is particularly beneficial in high demand applications like rotator cuff, ACL and Achilles repair where the re-tear rates far exceed an acceptable norm for surgeons or patients.”
There’s currently a range of standards for soft tissue repair, depending upon different applications. In rotator cuff, Rotation Medical (acquired by Smith & Nephew) has had success with their REGENETEN bioinductive implant, while decellularized dermal products have fallen off in use in recent years.
Conroy believes that the Embody approach can generate similar or superior results to REGENETEN with some other advantages, including on the economic side, making them a strong competitor in the rotator cuff space.
“Our novel additive manufacturing process (BioSpin™ hybrid electrospinning/pneumatospinning) combines clinical-grade collagen and Poly (D, L-Lactide) (PDLLA) to produce a controlled, highly aligned, cell-infiltration-friendly microstructure with controlled degradation profile, tailored fiber diameter, specific pore size and mechanical properties that mimic native tissue,” he said. “The materials are engineered based on the clinical application and the devices are carefully tailored to address clinical needs for each indication.”
Although many players occupy the biomaterials space, Embody employs a proprietary approach to creating collagen-based solutions through precise, controllable, scalable and novel additive manufacturing processes.
“We are taking a unique, bottom-up approach through advanced biofabrication techniques to create nano, meso and macroscale structures, enabling us to break through the traditional trade-offs of conventional biomaterials like porosity vs. strength,” Conroy said. “This allows us to tailor the attributes of the material to become a bioengineered analog to the human tissue we are looking to repair, like a tendon, for example.”
Going to Market
Embody plans to submit to FDA later this year and launch their first product, the TAPESTRY® Biointegrative Scaffold for Achilles and rotator cuff repair, early in 2020. They’ll start with Tapestry in multiple indications and advance their ACL program in parallel.
“Beyond these priorities, we have two opportunities before us,” Conroy said. “First, we are moving toward more complex implants preloaded with clinical grade cells and other ECM-like components as more complex orthopedic regenerative tools. Second, we have applications beyond orthopedics that we plan to advance.”
Much of Embody’s development to date has been recognized and funded by DARPA (Defense Advanced Research Projects Agency) due to the importance and injury rate of elite soldiers. Across the military, soft tissue damage is one of the most prevalent types of injuries that all servicemen and women face, comprising 50% of all injuries in the armed forces.
“We’ve built Embody with almost $18 million in non-dilutive funding from DARPA and other sources, and raised our first investor funding in 2018,” Conroy said. “We have a really good chance of being the first company ever to get an FDA clearance out of one DARPA contract. We are less than 12 months away from our FDA clearance and commercial launch, so we really are exhibiting Series B progress for a company just seeking our Series A fundraising. Our Series A investors will allow us to generate strong clinical evidence and commercial success in 2020 and beyond.”
Embody is finishing their pivotal animal study for 510(k) clearance, and plans to launch first human studies in Achilles and rotator cuff next year. ACL applications are likely coming in 2022 through a 100% collagen resorbable internal brace. Beyond that, they’re looking to expand further.
“I get excited because orthopedics is our test bed, but the key is that the trends in our industry are all toward using biologics and biologic-stimulating materials not just in orthopedics, but in areas like wound, general surgery, hernia, cardiovascular and nerve repair,” Conroy said. “Long-term, we’ve got a technology platform with broad applicability in regenerative tissue applications that will allow us to expand.”
Conroy looks at the future with clarity and optimism, confident in his team while concentrating on what needs to be done to drive success.
“Every day we are facing new challenges and I am delighted to have such a capable and strong team that continues to overcome and break through those challenges,” he said. “Clearly, our focus is on generating compelling clinical data and executing the steps required to drive market entry and revenue growth. This is our greatest challenge as we execute our 2019-2020 plan.”
HT
Heather Tunstall is a BONEZONE Contributor.




