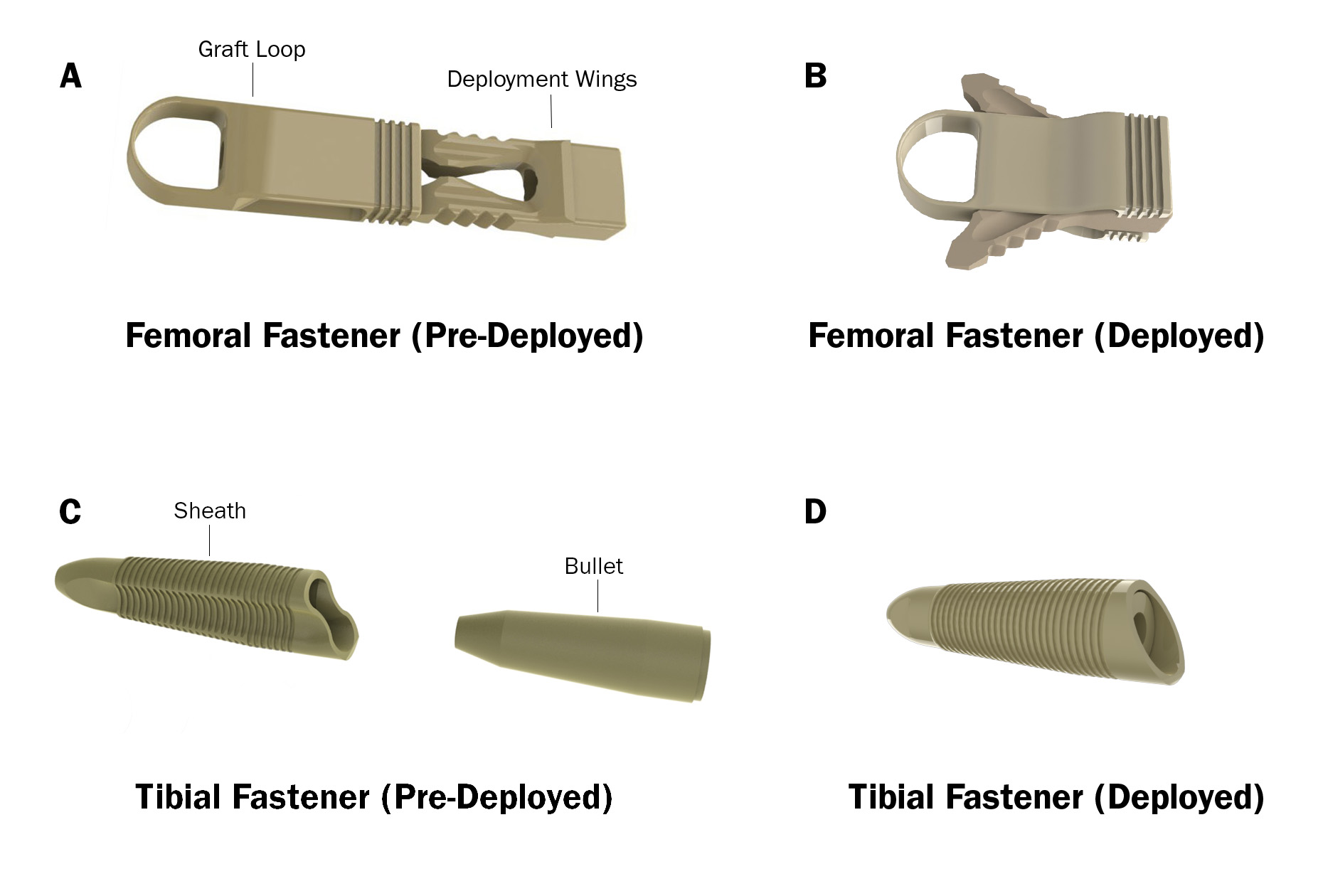
MedShape reached a definitive agreement for ConMed to acquire the ExoShape® Anterior Cruciate Ligament (ACL) fixation system. The transaction includes femoral and tibial soft tissue fasteners for fixation of soft tissue grafts in reconstruction procedures.
ExoShape products have been in use since 2011. In 2014, MedShape received FDA 510(k) clearance for its femoral fixation device that was the first to be constructed completely from PEEK Altera® shape memory polymer. The ExoShape system overall is reported to be the first all-PEEK system to offer a non-rotational deployment and interference fixation approach for ACL reconstruction.
In 1Q17, ConMed posted Arthroscopy/Soft Tissue Repair revenue of $103.8MM, -1.4% compared to 1Q16, and the company has just announced 2Q17 revenue of $105.6MM, -0.3% vs. 2Q16. ConMed’s turnaround initiatives include a revamp of marketing and a focused list of R&D projects and priorities. Additionally, the company seeks to bolster revenue via launch of the CuffLink Double Row rotator cuff repair system, Trinity 3-in-1 camera and new shaver blades, as well as through licensing agreements with Bovie Medical and KFx Medical.
Moving forward, MedShape seeks to increase investments in its core foot and ankle products for the double-digit-growth ankle repair market.


ExoShape® Anterior Cruciate Ligament (ACL) fixation system
Photo courtesy of MedShape




