Solar panels and hospital bed rails may have laid the foundation for future orthopaedic implants. One idea evolved from the study of surface technology, the other from a search for materials to inhibit microorganisms. Both use nanotechnology.
Nanotechnology is an industry buzzword, like additive manufacturing or robotics. Multidisciplinary technologies that experience success tend to build an urge within new surrounding industries to latch on to future possibilities and opportunities while the knowledge gap shrinks. That has created the buzz around nanotechnology in orthopaedics. “Nano” is allegedly being assigned to orthopaedic products without real use of the technology, an issue that could produce misunderstandings and hinder wide adoption in orthopaedics, according to some in the industry. Others believe that all conversation is good conversation.
And more than conversation is taking place. Major research is underway at the university and device company level, and new devices are expected to reach the market in the next five years.
Can nanotechnology move from buzzword to industry-shaping technology? Some think so.
The National Nanotechnology Initiative (NNI) defines nanotechnology as the understanding of control of matter at dimensions between 1 and 100 nanometers. To put size into perspective, a sheet of paper is about 100,000 nanometers thick and one inch contains about 25.4 million nanometers.
Nanotechnology has evolved from the engineering of individual particles to the development of whole systems—think millions of nanotubes to create an implant surface. With that shift comes the need for a greater understanding by industry and the general public of its capabilities, benefits and risks, says Gregory Nichols, Program Manager of the Nanotechnology Studies Program at ORAU, a 115-member university consortium. Nichols founded the program in an effort to map the nanotechnology landscape and educate on its advantages and implications. Since founding the program in February 2015, he’s identified 1,200 different entities, e.g., universities and companies, using and making nanomaterials in the U.S. Healthcare, including medical devices, is a primary focus of his.
The size and growth rate of the nanotechnology industry is disputed, with reports claiming revenue ranging from $300 billion to $3 trillion. NNI, a U.S. government-founded program, has a budget of $1.5 billion for 2015.
There is government and private money to be had for nanotechnology development. With that comes greater interest in research, development and commercialization, and most likely greater regulatory scrutiny.
Using nanoparticles of natural materials found in the body to impact tissue growth is the first best step to understanding nanotechnology in orthopaedics, because it removes a device and possible material toxicity from the equation, Nichols says. (Think hyaluronic acid.)
“The most important thing to keep in mind about nanotechnology is that it’s an interdisciplinary field,” Nichols says. “It’s important to bring different people into the research environment. It’s important for engineers to talk to public health people, industrial hygienists and business people to understand the challenges and barriers to market entry, early on in the development of products. That will be an important aspect of nanotechnology, moving forward. It is not an engineer in isolation coming up with a great idea. There are big issues that need to be tackled early.”
Generally, materials at the nano level enhance properties through higher strength, lighter weight and more control. Nanoscale materials vary greatly. Orthopaedic research ranges from hydroxyapatite and collagen to titanium and silver. The applications also vary. The projected evolution of nanotechnology in orthopaedics includes surface treatments, biologics, antimicrobial components and sensors. The main goals are to develop an implant that the body responds to faster and to develop an implant that decreases the chance for infection. While most companies are focused on spine, an advantage of nanotechnology is that it can be widely used throughout the body.
“To truly be nanotechnology, you have to have a physical structure designed to have features that function at the nano level,” says Dan Justin, President and CEO of Nanovation Partners. “In our case, we have engineered a surface treatment that forms ordered rows and rows of nanotubes with set diameter, wall thickness, spacing and height. We are able to precisely control these repeated features.”


Nanovation Partners’ TNT on Porous Ti6Al4V Structures
Nanovation is working with OEM and supplier partners that will apply the controlled anodization process to existing titanium alloy implants and to coated PEEK, ceramic, CoCrMo or zirconium implants. The nano surface treatment, which evolved from solar panel research by various groups and biomaterials research by Sungho Jin, Ph.D., at the University of California San Diego, can be applied to any metal surface that can be anodized, whether for a hip, knee, spine or other implant. The porous tubular array nanostructure encourages bone cells to adhere to the implant surface 300 percent to 400 percent more than with traditional roughened macro and micro structures alone, thus significantly reducing the need to cement the implant.
“The bonding process is triggered at the nano level,” Justin says. “The nanosurface mimics the structure of the outer membrane of the stem cells that are already floating around at the implant site, so the cells attach to the implant as they would attach to other like cells. The stem cells quickly spread out on the implant surface and differentiate into whatever cells are close to the nanotubes, whether an osteoblast cell if it is near bone, or a chondrocyte cell if it is near cartilage; then those cells continue to bond to the implant.”
The technology has been validated successfully in rabbits, pigs and sheep.
“We’ve seen nine times greater osseointegration bond strength in the implants that have our nanotubes on them than the implants that don’t,” Justin says. “We also see faster cell differentiation—the bonding and bone mineralization happens weeks faster than it would without nanotubes. We’re interested in allowing patients to walk on or fully use their implants faster under normal loading. We’re trying to speed that up not by days, but by weeks and perhaps months.”
Nanovation Partners, with offices in California and Florida, is working with OEMs to apply its patented anodization technology to the next generation of Class II devices. The same nanotube technology was licensed from UCSD by a dental company and is now used on a 510(k)-cleared dental implant. Nanovis, based in Indiana, has a similar technology platform that it plans to commercialize first with a nanotube surface treatment and then an antimicrobial one. Both companies see the ability to forge a regulatory path with each generation of product.
“The promise of a truly directed nanotechnology, not random, but trying to direct a response the size of a protein, is really exciting,” says Matt Hedrick, CEO of Nanovis. “To think about using that technology to regenerate soft tissue or apply to spine—that is a wonderful pathway for science, and it’s a real human benefit.”
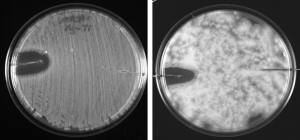

Methicillin-resistant Staphaureus (MRSA) and Aspergillus flavus (left to right)
The antimicrobial component of nanotechnology is a major focus of university research, says Rohan Shirwaiker, Ph.D., Assistant Professor in the Edward P. Fitts Department of Industrial and Systems Engineering at North Carolina State University. Shirwaiker, who recently made headlines for his battery-activated nanotechnology device, received the Best Young Investigator Research Poster Award from the American Academy of Orthopaedic Surgeons (AAOS) and Orthopaedic Research Society (ORS) in 2014. A portion of his research is focused on electric currents, through the use of a battery, releasing silver ions from a coating that would stave off infection.
“It is a much more effective way of controlling how many ions you are releasing, which then controls how fast you kill bacteria,” Shirwaiker says.
Shirwaiker’s Ph.D. dissertation looked at electrically activated silver as a material for hospital bed rails and doorknobs to repel bacteria. That technology is being commercialized. His research on implantables is still in the early stage. Through early rat tests, he is working to minimize the toxicity of the silver in the body.
“With any material, especially when you’re talking about nanoscale, ions and making things smaller, you have to be extremely careful in that while these things can kill bacteria and bad germs, they can also affect the human cells,” Shirwaiker says.
Shirwaiker sees the first use of the technology for infections during joint replacement. The implant would be removed; his implant would be put in until the infection clears and a new implant would be placed.
He eventually sees the use of nanotechnology evolving into sensors being placed into a hollow implant in order to send data to surgeons to detect items like proper load and infection rates. Commercialization of such technology is more than a decade away.
Nanotechnology is not a casual effort; it takes disciplined science. The little data that has been collected on nanotechnology in the orthopaedic industry is one of its hindrances to adoption.
“We have to be cautiously optimistic, because for some things, nanomaterials can work really well, but a lot of the side effects are not well-understood,” Shirwaiker says. “People know the benefits of nanoscale. They’re still trying to figure out what side effects there could be.”
Companies in pursuit of nano-component implants stress their need to tell a well-planned, data driven science story in order to receive regulatory clearance and hospital and surgeon approval. Titan Spine, which uses a nanosurface on its titanium spinal implants, has heavily promoted scientific papers on its implant to show both clinical evidence and to defend against competitors whom it says are using misleading nanotechnology claims that aren’t cleared by FDA.
FDA has been fairly mute on the topic of nanotechnology compared to other government agencies, like the Environmental Protection Agency, Nichols says. FDA refers to nanotechnology as “emerging,” but has not made a judgment as to whether it is inherently safe or harmful.
The Agency is conducting robust research to assess its safety and effectiveness and has started to issue product-specific draft guidance in relation to nanotechnology. Thus far, the guidance has been limited to foods and cosmetics.
Regulatory bodies haven’t taken strict action on nanotechnology because there isn’t enough data or reason to, Nichols says. But that could change as nanotechnology scales.
“We’re in a regulatory purgatory,” he says. “Some people think we should do more, but we don’t have the information to determine if we should do more. Everyone is holding their breath waiting, and I think we’ll be waiting for a while.”
The other major hurdle for companies will be educating the healthcare system, Nichols says. Nanotechnology is a complicated topic, and many industries face the need to overcome marketing gimmicks associated with its rise in popularity.
Companies truly manufacturing implants with nanotechnology will need to be armed with clinical and economic data and intellectual property to be successful.
“I’m focused on bringing our technology to the marketplace and not worrying about what other folks call it,” Justin says regarding the buzz surrounding nanotechnology. “When you start looking at things at a small level that have unique characteristics, it’s important to be scientific and not random about it. When you control the surface energy, the material properties and the surface shapes at the nano-level, that’s when exciting things happen.”
Photos courtesy of Nanovation Partners and Dr. Shirwaiker
CL
Carolyn LaWell is ORTHOWORLD's Chief Content Officer. She joined ORTHOWORLD in 2012 to oversee its editorial and industry education. She previously served in editor roles at B2B magazines and newspapers.




