In a move that complements its sports medicine portfolio, Smith & Nephew (SNN) acquired Rotation Medical for $125MM in cash, with up to $85MM in potential additional performance-based milestone payments. The transaction is expected to close by year-end and be earnings-neutral in 2018.
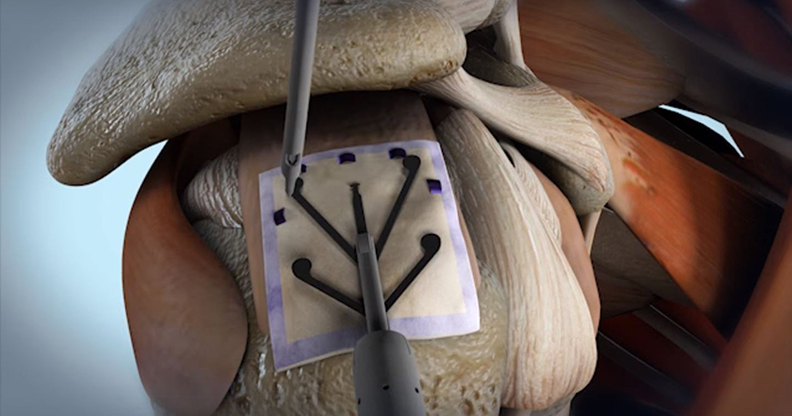
SNN is the second-largest arthroscopy/soft tissue repair company, deriving more than $1.2 billion or ~37% of its revenue from the segment, according to ORTHOWORLD estimates. SNN’s recent product launches have focused on the shoulder. The Rotation Medical acquisition allows SNN to add a collagen-based bioinductive implant for rotator cuff repair to its portfolio. The implant induces growth of tendon-like tissue and may prevent tear progression, as well as reduce the incidence of re-tearing. It comes with disposable arthroscopic instruments and can be used alone to treat partial tears, or with SNN’s anchors/sutures to supplement complex repairs.
The bioinductive implant received FDA 510(k) clearance and launched in 2Q14. Interim study results indicated that patients receiving the implant to treat rotator cuff disease reported statistically significant improvement at six months, vs. those undergoing standard treatment at two years.
In June 2014, we spoke with Martha Shadan, President and CEO of Rotation Medical, and we recap a portion of that conversation below. “Our goal was to generate a stronger, more natural repair of the torn tendon rather than mechanically or artificially augment it,” Shadan said. “We’re focusing on the biology and helping the body to repair itself. We believe that this will lead to a more durable repair of the degenerative tendon.”
Please describe Rotation Medical’s bioinductive technology and how it works.
Ms. Shadan: The system comprises a proprietary reconstituted bovine bioinductive implant and a set of disposable instruments that enable the procedure to be performed easily either through arthroscopy or as a mini-open procedure. Rotator cuff disease is a progressive disease—small tears propagate and become larger and more severe over time. Also, rotator cuff tears do not heal on their own. The degeneration of the tendon is believed to be caused by micro strains on the tendon, which prevent the body from healing.
Analysis has shown that if you can induce a layer of new tissue that augments the tendon or thickens it, you can achieve a substantial reduction in those strains. As the strains are reduced, the natural healing process takes place. It’s with this basic science that we set out to develop our bioinductive implant. It’s designed to be highly porous and low modulus. It’s not designed to provide strength; rather, it’s designed to rapidly induce new tendon tissue growth. It’s the new tissue growth that biologically augments the tendon, rather than the implant itself.
Can you further explain the science behind the technology?
Ms. Shadan: It is a highly porous bioinductive implant. It allows for rapid ingrowth of fibroblast and blood vessels. Those fibroblasts and blood vessels mature into dense, highly-organized tendon-like tissue. That new tissue becomes a biologic augmenter.
What are the benefits of this system vs. the current standard of care?
Ms. Shadan: As it relates to the early stages of the disease, if a patient received treatment in addition to receiving acromioplasty, we are actually inducing new growth, which causes a healing of the tendon. Rather than just getting relief from the pain, the patient ends up with a more normal-looking tendon. In addition to that, the rehab would be exactly the same as if they were just relieving their symptoms. From that perspective, they get a whole new tendon with no additional rehab. In the later stages, in some cases you can use our technology instead of the normal suture and suture anchor repair. Again, by inducing the new tissue, you get a healed tendon and the rehab would be substantially less. Instead of four to six weeks in a sling and four to six months of rehab, it would be one to two weeks in a sling and maybe a month of rehab.
What trends are you seeing in the shoulder segment of the market?
Ms. Shadan: First of all, it’s an area of strategic interest and focus by many multi-national companies. It’s an area that is continuing to grow, and the growth is substantially better than some of the other orthopaedic areas. What we’re also seeing is increasing focus on technologies that induce growth or regenerate new tissue. There is a strong interest in trying to not only mechanically repair, but to generate tissue that is more normal and enduring than just relying on mechanical repair. In a study, “The Future of Growth & Innovation in U.S. Extremities Ortho Reconstruction” (Nov. 20, 2013), a number of physicians were polled and one of the biggest unmet clinical needs was innovations in technology that speed up the recovery process, such as biologics, grafts and other materials that can foster tissue growth and other healing. This is an area of strong strategic interest.
Since the publishing of Ms. Shadan’s interview, SNN has made several acquisitions in the arthroscopy/soft tissue space, including ArthroCare in 2014 and BST-CarGel’s cartilage repair product in 2016.
The Rotation Medical acquisition follows the announcement that SNN CEO Olivier Bohuon will retire in 2018, as well as reports that U.S. activist investor, Elliott Management, obtained a greater than 2% stake in SNN and, as one of the company’s top seven shareholders, could be pushing SNN to divest portions of its business to become a more attractive acquisition target. SNN’s leading position in the arthroscopy/soft tissue market makes the company as a whole a more valuable acquisition target and would not be a segment to divest.
Rob Meyer is ORTHOWORLD’s Senior Editor.




