Additive Manufacturing: Capability Report on Production-Ready Parts
Additive manufacturing has transitioned from a prototype-only mentality to production-centric techno...
08 February, 2021BONEZONE is owned and produced by ORTHOWORLD® Inc., a customer-centric media company focused solely on the global orthopedic market for more than 30 years. ORTHOWORLD’s core customers are executives and engineers from orthopedic device companies. It is through their voices that BONEZONE was conceived and brought to market in 2002.
BONEZONE is your foremost source on orthopedic product development.
Voice of customer drives our editorial decisions. We leverage our deep orthopedic industry knowledge, expansive network and input from our Advisory Board to keep content stimulating, relevant and useful.
Our editorial priority is to bolster OEMs’ product development initiatives by educating their engineers and executives on business-critical topics that fuel prosperity; competitive landscapes, pioneering advancements, market shifts, regulatory changes, human behavior, digital technology and more.
OMTEC®, the world’s only conference exclusively serving the global orthopedic manufacturing community serves as the “live” version of BONEZONE where advertisers often exhibit and readers often attend.
BONEZONE print magazine is accompanied by a digital ePub, Newsletters, a robust website and relevant webinars. The BONEZONE Supplier Directory, an exclusive network of orthopedic-focused suppliers, bolsters OEMs’ networks and expedites their initiatives.
OEM readers are actively seeking new suppliers and service providers with expertise in all aspects of product development. Every BONEZONE advertising offering is designed to build brand equity, generate leads and grow your OEM customer base.
OEMs trust ORTHOWORLD and its family of brands (ORTHOWORLD, OMTEC, BONEZONE). We look forward to welcoming you as an advertiser.

BONEZONE is mailed 5 times a year to orthopedic engineers around the globe. Pay $0 with promo code: WEBSITE
BONEZONE readers seek suppliers and service providers that offer a range of commercialization solutions, from prototypes to inventory management.




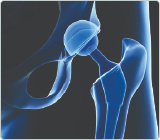



Additive manufacturing has transitioned from a prototype-only mentality to production-centric techno...
08 February, 2021Considerations for manufacturing robotic and navigated instruments and advice on finding a partner....
31 March, 2021Analysis on developing lattice structures, fatigue properties, optimized HIP, powder aging and quali...
22 June, 2021This white paper documents a thorough process capability study performed using 3D Systems’ laser pow...
25 June, 2021