Introduction
As the use of medical imaging in clinical trials and manufacturing quality assurance continues its rapid increase, orthopaedic device companies face an array of complexities. In many cases, imaging technologies (CT, MRI, PET, x-ray, etc.) can speed the process of proving product efficacy and safety. However, keeping track of the rapidly-changing imaging and image analysis technology landscape can be challenging.
To assist with the imaging components of their work, clinical trial sponsors often turn to imaging core laboratories or central imaging laboratories. These organizations help secure consistent, high-quality imaging data and ensure minimal variability through centralized radiologist image reading and analysis. They also guide sponsors in selecting the best combination of imaging modalities, designing and evaluating appropriate image acquisition protocols, providing study site imaging oversight and compliance and offering quality control and image data management, throughout.
An emerging alternative is use of an imaging contract research organization (ICRO) that employs a combination of imaging, image analysis, biomedical and software engineering expertise along with customizable software for image analysis, data capture and management. Custom-tailored image analysis algorithms can be used in lieu of time and cost-intensive manual processes, reducing guesswork, cost and time associated with preclinical and clinical studies. Particularly adept ICROs can leverage their expertise to translate the quantitative image measurement into valuable 2D and 3D marketing collateral to help study sponsors bridge the gap between product regulatory approval and market adoption. While the following is not an exhaustive list of ICRO critical features, it does provide a strong foundation for evaluating an ICRO to support your next preclinical or clinical study.
Operational Abilities
Some ICROs specialize in a specific therapeutic area, or in support of preclinical vs. clinical studies. On-site vs. off-site access to professional staff (e.g., radiologists and pathologists) to read and provide or validate image analysis outputs is an important consideration, as an on-site staff means the sponsor can expect better access to expertise, better turnaround time and fewer potential logistical concerns. Further, an on-site professional staff gives the ICRO greater control over the quality of the manually scored image data (1). On the other hand, on-site clinical staff can often translate into higher costs and reduced access to a broader range of clinical sub-specialty expertise.
One often overlooked aspect of an ICRO’s operational prowess is its ability (and willingness) to provide image acquisition and data management support and expertise to researchers, clinicians and technicians at remote study sites to optimize and standardize imaging equipment and protocols across multiple sites. Since clinical 3D imaging modalities have such low resolutions (relative to modalities like Micro-CT), an ICRO’s ability to help technicians fine-tune and optimize a CT or MRI scanner can mean the difference between “okay” and exceptional data. Given the inter-vendor and inter-model variability and complexity for a given imaging modality, it’s easy to see how resulting study data variability can become quite large if not dealt with prior to study initiation. Finally, as a result of the inherent differences in preclinical and clinical imaging modalities (e.g., resolution, image acquisition time, field-of-view, radiation dose, etc.), an ICRO with expertise in both can add a great deal of value by helping a sponsor successfully translate preclinical product efficacy outcomes into clinically relevant clinical trial efficacy end point measurements.
Out-of-the-Box Creativity
Few aspects are more important than the ability to preemptively identify problems, quickly design solutions and then implement those solutions efficiently and effectively. Creative thinking promotes problem-solving and innovation. For example, a common problem in orthopaedic studies utilizing CT or MR imaging over multiple time points is that the 3D images are not acquired in the same plane (e.g., sagittal versus axial) due to inconsistent patient or specimen positioning. Unless an ICRO can quickly digitally reorient and co-register these image volumes to the same plane and coordinate system, radiologists will have to manually account for this during image scoring. As a result, additional subjectivity, variability and labor are introduced into the data set. One solution would be to account for this when the team of readers are trained and standardized prior to the study beginning. It is also possible to design an image acquisition protocol to account for patient positioning, in addition to providing study site training and oversight. Alternatively, a technically advanced ICRO would anticipate this and develop study- and product-specific (e.g., drug, device, biologic) image co-registration algorithms to reorient the images prior to analysis by a reader or quantitative software.
Imaging Modality Diversity
If an ICRO offers a particular image acquisition modality, they need to be well versed in the science behind it. Diversity of experience with various imaging modalities is important, as is an ability to plan a study relative to the best combination of imaging modalities to achieve end points.
For example, an ICRO should be well versed in both 2D (e.g., ultrasound, X-ray, confocal and scanning electron microscopy) and 3D (e.g., CT, Micro-CT and MRI) imaging modalities. This enables the ICRO to offer multiple options (and combinations) for establishing optimal study end point measurements. This also demonstrates that the ICRO is not limited by a single imaging technique. Orthopaedic implant histology is often a costly and time consuming endeavor that requires a sponsor to sub-sample the entire available data set. To mitigate the time/cost burden, an ICRO should offer its sponsor the option to use Micro-CT in combination with a reduced amount of 2D histology. In addition to reducing data turnaround time and cost, this approach should yield data that is more quantitative and comprehensive (2).
Therapeutic Research Diversity
Having experience in multiple medical areas (i.e., orthopaedics, cardiology, oncology, etc.) lends a broad and valuable perspective from which to design and execute a study. For example, an orthopaedic spinal implant (e.g., disc replacement) clinical trial involving multi-planar X-ray, CT and MRI imaging over multiple time points would require very different imaging and image analysis controls than a preclinical study to evaluate an early-stage cancer drug whose image-based end point measurements focus on assessing angiogenesis at the cellular level. Understanding these details and how they can be manipulated and implemented early on in a study, can have a substantial impact on the timeline of a study and the quality of the final data.
Technology Implementation
ICROs use many different technologies to practice their craft. For example, among ICROs, a Picture Archiving and Communication System (PACS), image scoring and data tracking software and a secure “portal” (i.e., web-based) for managing, transferring and communicating important information (including raw and evaluated image data) among the ICRO, study site(s) and the sponsor, should be fairly universal. Some ICROs even use software engineering to custom-tailor image processing, analysis and visualization techniques on a study-by-study, product-by-product and disease-by-disease basis (3, 4, and 5). This enables the ICRO to quickly generate extremely objective quantitative data, and offer the sponsor a wider range of image measurement options with increased analysis precision and accuracy. (See Exhibit 1.) Regardless, what is important for an orthopaedic device company to consider is how an ICRO uses all of this technology and what it translates into for the sponsor. If the ICRO is particularly technology savvy, they should be able to leverage custom-tailored imaging analytics to reduce the cost, time, labor and variability associated with a study by either increasing the efficiency of a study, or by increasing the objectivity, precision and accuracy of the end point measurements such that fewer specimens (or patients) are required to demonstrate product efficacy.
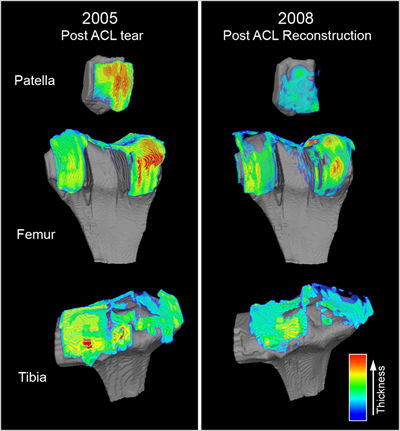
Exhibit 1: Technology Implementation at Work
Cartilage Repair Quantification. In this example, custom-tailored magnetic resonance (MR) image analysis algorithms were developed, validated and employed to quantitatively measure and visually map the cartilage thickness across the patella, femur and tibia for a cohort of patients post-ACL reconstruction. In this study, control and treatment patient groups underwent MR imaging at 0, 6, 12, 24 and 36 months post-repair wherein the defect size and cartilage thickness were quantified at each time point. At the end of the study, the MR image data for all 5 time points for each patient were co-registered in 3D space such that changes in defect size (“defect repair/healing,” data not shown) and cartilage thickness (evidence of delamination) could be quantified and visualized. This approach provided the sponsor and study site investigators with comprehensive quantitative insight into their product’s clinical performance.

Track Record
Finally, it is important to consider the experience of the ICRO as a whole (for instance, with what other sponsors have they worked?), as well as the people involved in planning, designing, evaluating and executing the study. The ICRO should be able to confidently answer specific study questions and provide references that will give an honest account of their experiences. An ICRO should have a track record for successfully working with a sponsor to present and defend study data to regulatory agencies. For example, in the U.S., an ICRO should be willing to attend pre- and post-study meetings with FDA to present, review and defend both study data and their approach for obtaining that data. To this end, an ICRO should have access to regulatory experts with study-specific scientific experience.
An ICRO should also have a track record for working with sponsors to identify and solve ongoing imaging-related problems that crop up. In the unfortunate event that something does go awry (e.g., insufficient image quality prevents image analysis), the ICRO should embrace and quickly and efficiently solve the issue. It is unacceptable for an ICRO to “pass the buck” or simply state that a problem is unfixable without first investing the time and care to diagnose the problem and provide either a solution or an explanation for why the issue cannot be resolved. In short, an ICRO should treat any sponsor’s study as if it were their own.
When the success of an orthopaedic study is at stake, sponsors want an ICRO that can help accelerate their journey from bench to bedside. A sponsor should be confident that the ICRO can support their imaging-related efforts in every capacity. Investing the time and effort up front to vet your ICRO will pay dividends over the long haul in the form of fewer headaches, better data and shorter timelines.
References
1. Taranto, R. 2007. Imaging CROs and their impact on clinical trials. Drug Development and Delivery: Specialty Pharma. Vol. 7 (6): 72-76.
2. Jimbo R et al. 2011. Histological and three-dimensional evaluation of osseointegration to nanostructured calcium phosphate-coated implants. Acta Biomater.
3. Hildebrand, T and Ruegsegger, P. 1997. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc. Vol. 185: 67-75.
4. Saito, T and Toriwaki, JI. 1994. New algorithms for Euclidean distance transformation of an n-dimensional digitized picture with applications. Pattern Recognition. Vol. 27: 1551-1565.
5. Tsao, YF and Fu, KS. 1981. A parallel thinning algorithm for 3-D pictures. Computer Graphics and Image Process. Vol. 17: 315-331.
Brett Hoover has more than ten years of experience in Biotechnology and Life Science as a researcher, product manager and commercialization specialist. Prior to joining ImageIQ, he served as a product manager for the Clinical Tissue Engineering Center and as a commercialization specialist for the Armed Forces Institute for Regenerative Medicine, both of which are located at Cleveland Clinic.




