
In an effort to promote innovation while protecting the population at large, the US Food and Drug Administration (FDA) has for several years been on a course to revise the existing regulatory pathway surrounding the 510(k) process for medical devices. One topic of concern focuses upon clinical data. Historically, this has plagued device manufacturers who risk submitting their 510(k) only to discover afterwards that clinical data will be required, or that perhaps even if they submitted clinical data, it was not enough. FDA’s efforts to review the current status of the program are intended, among other things, to result in changes that will provide prospective answers to device manufacturers regarding whether or not clinical data is required, and if so, to what extent. The purpose of this article is to establish the current status of the 510(k) process with regard to clinical data requirements, and then to begin a discussion regarding some considerations to be given when conducting a well-controlled clinical study, if indeed clinical data is required.
Current Status of 510(k) Clinical Data Requirements
The determination of whether or not a device clinical trial is required is largely based upon a risk stratification of the device, as illustrated in Exhibit 1 below. Minimal risk or Class I devices do not require a clinical trial, whereas some intermediate risk devices (Class II) and all substantial risk devices (Class III) do require a clinical trial.
Exhibit 1: Medical Device Risk Classification Chart
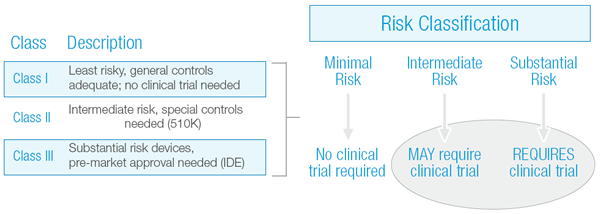

If a device falls into the Intermediate Risk category, there is a possibility that clinical performance data may be required. Unfortunately, whether or not clinical data is needed, or the extent to which it is needed, has not always been clear.
Beginning in September 2009, the Center for Devices and Radiological Health (CDRH) established two internal committees to review the 510(k) program, including the 510(k) Working Group and the Task Force on Utilization of Science in Regulatory Decision Making. In addition, they commissioned the Institute of Medicine (IOM) to conduct an independent review of the 510(k) process in parallel with their own investigation.
In August 2010, the internal committees released their preliminary reports and recommended actions, including proposals surrounding the requirements for clinical data. Taking those recommendations along with public comment into account, FDA released its plan for implementing 25 actions in January 2011. In that report, FDA deferred for further independent evaluation by the IOM seven areas for additional consideration, including the concept of whether or not to establish a “Class IIb” classification of medical devices for which clinical information would typically be required to support a submission. While lack of time did not permit the IOM to fully investigate this area, in July 2011, Dr. David R. Challoner, Chair of the IOM’s committee, sent a letter to Dr. Jeffrey Shuren, Director of the CDRH, recommending that the 35-year-old program be eliminated in favor of “a more rational medical device regulatory framework.” A timeline of these important dates is illustrated in Exhibit 2.
Exhibit 2: The 510(k) Timeline (Click here to view exhibit as a PDF)
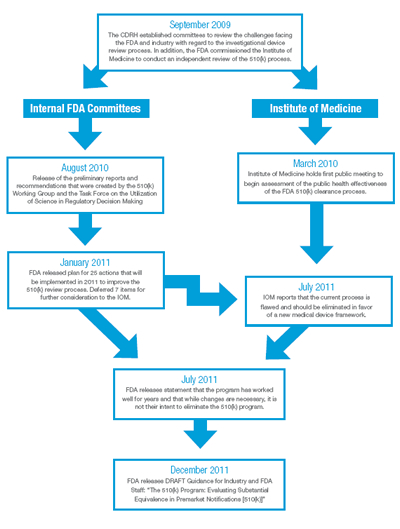

Shortly after the IOM’s recommendation, FDA released a statement indicating that, while changes are necessary, it was not their intent to eliminate the 510(k) program. On December 27, 2011, FDA issued a Draft Guidance for Industry and FDA Staff entitled “The 510(k) Program: Evaluating Substantial Equivalence in Premarket Notifications [510(k)]” which is intended to “enhance predictability, consistency and transparency of the 510(k) program.”
Is a Clinical Trial Needed??
How does one determine the responsibilities regarding clinical data requirements? As of January 2011, FDA requires clinical data in approximately ten percent of all 510(k) submissions. Heather Rosecrans, the Director of the 510(k) Staff in the Center for Devices and Radiological Health for FDA, explained in an overview of the 510(k) process presented in January 2011 that clinical data is required when there is an “Important difference with the predicate device, e.g., new indication for use or new technology.” The draft 510(k) guidance document released on December 27, 2011 goes further by describing situations that would likely lead to the requirement for clinical data, including new or modified indications for use, technological differences and/or non-clinical testing methods that are limited or inappropriate because of the indications for use or device technology. In addition, the guidance states, “In many cases, the clinical data necessary to support a 510(k) involve a relatively small number of patients and may involve a simpler study design than is necessary to support a premarket approval application.”
Considerations if Clinical Data is Needed to Support a 510(k) Submission
While more clarity is forthcoming regarding the clinical data requirements for 510(k) submissions, it is important to know what the requirements are in the event that a clinical study is needed. Rosecrans explained that when clinical data is required, the requirements for conducting the study are the same as those for conducting an Investigational Device Exemption study as outlined in 21 CFR 812 (2011).
Knowing the regulations to be followed and the responsibilities for sponsors outlined within those regulations is important in developing a strategy for compliance. Ensuring that the clinical study team has an understanding of the regulations is important throughout the clinical study process. Upfront training on the regulations for everyone involved will set the stage for a well-controlled, compliant clinical study. The end result is a clinical study in which patients are protected and the resulting data provided to the FDA to support the 510(k) submission has integrity.
Sponsor Responsibilities
21 CFR 812 outlines general and specific responsibilities of sponsors and serves as a guide for general conduct of clinical studies. Requirements regarding the contents of the investigational plan, the report of prior investigations, labeling and other requirements are described in detail in Part 812 and fall outside of the scope of this article. Specific responsibilities of sponsors are described in 21 CFR 812.40, which include selecting qualified investigators, providing investigators with necessary information, ensuring proper monitoring, and ensuring that Independent Review Board (IRB) approvals are obtained prior to enrolling human subjects. In addition, responsibilities regarding record keeping are contained in 21 CFR 812.140 and 21 CFR 812.145. All of these responsibilities boil down to protection of human subjects, and are presented in more detail as follows.
Selecting Qualified Investigators
It seems logical that if an orthopaedic device is being researched, a psychiatrist would not be sought to be an investigator. 21 CFR 812.43 specifies that an investigator should be selected based on training and experience and should be qualified to operate as an investigator in the clinical study. Therefore, investigators involved in the clinical study should have relevant experience. To document this, sponsors may collect their curricula vitae, medical licenses or other evidence of the investigator’s background which demonstrate experience in the area being studied. Depending on the complexity of the device, it may also be necessary to document specific training in device preparation and implantation, for instance. In addition, the sponsor should collect a signed agreement from the investigator stating his or her intent to comply with the appropriate regulations. Minimal agreement contents are summarized in Table 1.


Additional items to consider building into an agreement may include expectations with regard to communication, meeting attendance, data submission timelines, essential document submission, and financial compensation, among others.
Informing Investigators
Sponsors are required to provide investigators with copies of the protocol and any other information, including previous experience with the device that would be necessary for him or her to conduct the study (21 CFR 812.45). Sponsors should document how this information was disseminated and the dates on which the investigators were given the information. Changes to the protocol or modifications to the device should be communicated throughout the study, and documentation of such should be maintained.
In device studies, the influence of physician technique may be very high, depending upon the type and complexity of the device being studied. Carefully and safely implanting an artificial knee, for instance, relies on expert knowledge of appropriate surgical technique, and may require upfront training on bench top or animal models. Troubleshooting in the event of problems would also be critical. For this reason, ensuring that investigators have adequate information to conduct the study may require much more than just providing them with a protocol. A decision regarding the extent of training to be required for the study should be made at the start. This may include hands-on training with another physician or engineer; it may require the presence of a proctor during a certain number of cases, or other methods suitable for that particular device.
Ensuring Proper Monitoring
The purpose of monitoring is to protect patients by ensuring data integrity and compliance with applicable regulations. In order to ensure that the study is properly monitored, the sponsor must first select qualified monitors. 21 CFR 812.43 (d) indicates that monitors must be qualified by training and experience to monitor the study. This means that the monitors have clinical experience or have received training in the area being studied, and that they have been trained or have operated as a monitor. Documentation of the monitor’s qualifications should be maintained by the sponsor to demonstrate compliance with this requirement.
The level of monitoring oversight that is implemented during the study may be based on the risk associated with the device, including both the risk to patients and to the company. If this is a complex device, or one critical to the sponsor’s pipeline, it may be prudent for the sponsor to take a more conservative approach to monitoring, sending monitors to the sites more often to review subject records and regulatory documentation to ensure that the study is being conducted properly. If it is not a complex device or is less critical to the sponsor’s pipeline, perhaps the monitoring plan includes more remote review of data and fewer onsite visits. Either way, a monitoring plan should be implemented prior to the study, and monitoring procedures that are based in the aforementioned regulations should be developed and followed.
Monitoring may be done remotely or onsite, and can be performed by internal staff or a third party (i.e., independent monitors or through a contract research organization). Again, the decision regarding which method of monitoring to use may largely be based on a risk assessment of the study in question. In addition, resourcing internally may dictate that an outside firm be used if the sponsor intends to take a conservative approach to monitoring. Regardless, areas to examine through the monitoring process include: the informed consent process, eligibility, protocol compliance, source data verification, device accountability and regulatory compliance, among others. Additional insight into FDA’s current thinking on monitoring approach can be found in a recently-released draft guidance, entitled “Guidance for Industry – Oversight of Clinical Investigations – A Risk-Based Approach to Monitoring.”
Ensuring IRB Approval
As described in 21 CFR 56.102 (23) (g), the main purpose of IRB review “is to assure the protection of the rights and welfare of the human subjects.” Whether using a central or a local IRB, IRB approval is needed prior to beginning any clinical study activity, as required in 21 CFR 812.42. While it is the investigator’s responsibility to seek approval from the IRB, it is the sponsor’s responsibility to ensure that approval has been obtained.
Generally speaking, most IRBs require specific paperwork be completed with the initial submission, outlining such items as protocol, consent form to be used, consenting practices to be employed, eligibility criteria, staff who will be working on the study, procedural and follow-up requirements, populations that will be included in the study and other important items. While some sites complete this paperwork without assistance from the sponsor, the sponsor’s involvement in this process can help reduce the time to approval. Specifically as it relates to the informed consent document, the sponsor’s approval of the document prior to IRB approval would be beneficial. Should additional changes be required to the consent form after IRB approval, the study may be delayed until such a time as the IRB meets again. In some cases, this could be monthly, bi-monthly or quarterly.
Consideration should be given to implementing procedures at the sponsor level whereby study devices are not shipped until IRB approval is received. Documentation of the approval should be maintained both in the site’s and the sponsor’s files.
Maintaining Adequate Records
FDA has requirements for record keeping for both investigative sites and sponsors. The sponsors will not only be responsible for ensuring that their files are current and complete, but also for ensuring, through monitoring or other methods, that the investigator is keeping records as required in 21 CFR 812.140. Helping the site by setting up a study binder complete with sections for each required type of documentation would be beneficial in assisting with compliance. In turn, many sponsors maintain mirror files, requesting that sites copy them on all pertinent study documentation. While the approach may be different, the regulatory requirements remain the same. Documentation that is specified as a sponsor requirement to maintain is described in Table 2.


Conclusion
Running a clinical study could be a monstrous undertaking for a company that historically has not had to produce clinical data for 510(k) submissions, and this article only begins to touch the surface. Subsequent articles will address issues such as investigative site selection and responsibilities, global considerations, informed consent, financial disclosure/conflicts of interest and all of the corresponding documentation requirements for each issue. With the hope of more predictability and transparency on the horizon, device manufacturers may want to consider developing a working knowledge of what it takes to run a clinical study. Understanding the regulatory framework within which they need to operate, choosing competent investigators, providing training both to their internal staff and their investigative sites, implementing an appropriate level of oversight and maintaining all the pertinent documentation will allow companies to feel confident in the clinical data that is supporting their 510(k) submission. More importantly, sponsors can feel confident in knowing that the human subjects on the other side of that device were protected.
REFERENCES
Department of Health and Human Services: Center for Devices and Radiological Health. (2011). Accomplishments: CDRH Plan of Action for 510(k) and Science. www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHReports/ucm276286.htm.
Department of Health and Human Services: Center for Devices and Radiological Health. (2011). 510(k) and Science Report Recommendations: Summary and Overview of Comments and Next Steps. www.fda.gov/downloads/AboutFDA/CentersOffices/CDRH/CDRHReports/UCM239449.pdf.
Department of Health and Human Services: Center for Devices and Radiological Health. (2011). Guidance for Industry. Oversight of Clinical Investigations – A Risk-Based Approach to Monitoring. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM269919.pdf.
Department of Health and Human Services: Center for Devices and Radiological Health. (2011). Plan of Action for Implementation of 510(k) and Science Recommendations. www.fda.gov/downloads/AboutFDA/CentersOffices/CDRH/CDRHReports/UCM239450.pdf.
Department of Health and Human Services. Code of Federal Regulations – Title 21 – Food and Drugs; Chapter I – Food and Drug Administration; Subchapter H – Medical Devices: Part 812 – Investigational Device Exemption. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=812&showFR=1.
Institute of Medicine of the National Academies. (2011). Medical Devices and the Public’s Health: The FDA 510(k) Clearance Process at 35 Years. www.iom.edu/Reports/2011/Medical-Devices-and-the-Publics-Health-The-FDA-510k-Clearance-Process-at-35-Years.aspx.
Rosecrans, H. (2011). 510(k) Overview. www.fda.gov/downloads/MedicalDevices/ResourcesforYou/Industry/ucm126293.pdf.
Sandra Maddock, RN, BSN, CCRA is the President/Chief Executive Officer of IMARC Research, Inc., a contract research organization based in Cleveland, Ohio. Founded in 1999, IMARC specializes in providing third party clinical trial oversight for the medical device industry via their monitoring, auditing and GCP training services. Sandra may be reached at SMaddock@imarcresearch.com
IMARC Research, Inc.
440-801-1540 (phone)
www.imarcresearch.com
Continue learning from Ms. Maddock: attend her educational session at OMTEC 2012 in Chicago.
Running a Clinical Trial: How to Navigate Through the Regulatory Maze
Wednesday, June 13, 1:30 pm – 3:00 pm
Whether you are a company owner, project manager, engineer or assistant, you may be called upon to run a clinical trial or be involved, in some way. Attendees will learn about the FAIR Shake™ concept which takes a complicated set of regulations and breaks it into four very simple questions to enable you to make educated decisions as you move through a trial.
The session begins with a “current climate” look at FDA, then moves to the “how to” of navigating through regulations, specifically targeting what that will mean to attendees who will return to research settings. This presentation will be highly interactive, using case studies with an orthopaedic focus.




