
“There’s always room for Jell-O.” —Peter Venkman, Ghostbusters II, 1989.
The human body is an intricate combination of hard and soft tissues, the interaction of which leads to its amazing flexibility and durability. To date, the vast majority of implantable biomedical devices use rigid materials composed of thermoplastics, epoxies and metals. The use of rigid materials has been very successful in some cases, such as total hip and knee replacements, in which the general strategy has been to engineer mechanical joints that can respond to the mechanical loads and biomechanics while minimizing wear. Currently missing from this strategy is the dynamic response of the soft tissue in joints, which provides conformable surfaces, improved comfort to the patient and potentially improved range of motion. Nature has already provided such a surface in the form of cartilage. Researchers have been striving to understand both the nature of cartilage and how to mimic it in a synthetic manner to allow the development of soft-solids biomedical implants for large and small joint applications. Additionally, approaches to reinforcing soft tissue require the use of a material with similar physical properties to native tissue, and preclude the use of rigid synthetic materials. Hydrogels are one possible solution.
Hydrogels in the Body
Nature has made extensive use of structured and inhomogeneous soft solids to provide vital roles. Mucus, vitreous humor, cartilage, tendons and blood clots are all forms of material known as hydrogels. Hydrogels are characterized as being neither solid nor liquid, but rather a combination of the properties of both. Structurally, a hydrogel is a 3-dimensional network of material that confines and supports water, but is not soluble in water, although the individual polymers that make up the hydrogel usually are. Hydrogels will typically contain between 50 percent to 90 percent water, depending on the formulation and degree of crosslinking. Gelatin is the most well-known example of a hydrogel, and is a proteinaceous structure derived from collagen extracted from animal tissue. The proteins form a 3-dimensional structure that are “crosslinked” together to yield the continuous, water-containing structure of the hydrogel. The vitreous humor of the eye and cartilage both contain protein hydrogels derived from collagen, which highlights that although chemically almost identical, markedly different properties can be obtained by varying the structure of the crosslinked network, ranging from a transparent low viscosity fluid to a tough, load-bearing construct.
The key to the success of hydrogels in the body is their viscoelasticity and permeability. Viscoelasticity is the behavior that results from being halfway between a rubber (in which energy put in is instantly restored – think of bouncing rubber balls) and water (in which inputted energy is lost to drag – think how fast a stone stops when it stops skimming). The crosslinked, polymeric structure of hydrogels results in an elastic response to rapid deformations, whereby the ability to transport fluid results in a compliant response to slow deformations, while still maintaining their shape over long periods. Viscoelasticity is one of the keys to cartilage’s success as a bearing surface. Cartilage is conformable under static loads, but maintains is shape and elasticity when subjected to impact loads. Viscoelasticity is not the only important property of cartilage, however. The hydrogel nature of cartilage results in the generation of a lubricating layer of fluid on the surface of the cartilage, thereby reducing the coefficient of friction on the bearing surface. Additionally, the permeability of hydrogels allows them to be populated by cells and transport nutrients and solutes across spaces in the body. Plasticized rubber, a synthetic engineering material, can have similar mechanical properties to cartilage, but is totally impermeable to aqueous-based fluids. Hence, it has neither the lubricating layer nor the ability to populate cells, both properties found in cartilage, which limits its utility in this application area. Synthetic hydrogels do have possibilities in this area.
Forming Hydrogels
Hydrogel formation can be grouped into two broad classes: those formed by chemical crosslinking, and those formed by physical bonds. Chemically-crosslinked hydrogels have an analogy to epoxies, which are 3-dimensional constructs typically made of hydrophobic polymers that have covalent bonds between chains. These hydrogels can be formed directly from monomers, which is how most contact lenses are manufactured, starting from monomers including vinyl pyrrolidone (NVP), methacrylic acid (MA) and poly-2-hydroxyethyl methacrylate (pHEMA). Alternatively, the hydrogel can be formed by crosslinking hydrophilic polymer chains with a crosslinking agent or by radiation, or by hydrolyzing a hydrophobic network. Another example is polyethylene glycol (PEG) which is often used for drug delivery applications.
Although great advances have been made in developing chemistries to allow sufficient safety profiles, it is an intrinsic weakness of these systems that: 1) the reaction that generates the crosslinks results in a heat increase (exotherm) as the bond forms; 2) there will be unused materials remaining which are reactive and potentially toxic; and 3) a bond forming reaction results in reaction by-products that may have undesirable safety profiles.
Hydrogels formed from physical bonds present a broad class of materials. These physical bonds can be formed by crystalline junctions, hydrogen bonding, phase-separation, ionic bonds or other associations. (See Exhibit 1.) The strength of the hydrogel depends upon the strength of these physical bonds and their density. As one example, triblock copolymers can be constructed with hydrophobic end blocks and a hydrophilic center block. When placed in water, the end blocks associate into tight bundles, and the hydrophilic blocks absorb water and expand. A 3-dimensional, water containing structure can form. These hydrogels are sometimes reversible, in that changing the conditions (temperature, pH, salinity) can sometimes permit the gel material to go back into solution.
Exhibit 1: Schematic Diagram of a Hydrogel Formed by Physical Bonds


A well-researched polymer, polyvinyl alcohol (PVA), readily forms hydrogels by a variety of mechanisms. While PVA hydrogels can be crosslinked by chemical (covalent) bonds, they are normally processed by creating hydrogen bonds resulting from the interaction of a hydrogen atom with an electronegative atom, such as oxygen. Although this bond is weak relative to the covalent bond, it can still be structurally stable, and is the mechanism used to stabilize folded proteins and give water its unique properties. These hydrogen bonds create crystalline junction points in the material, generating a hydrogel. Researchers will commonly form PVA-based hydrogels by repeatedly freezing and thawing a PVA-solution, which allows the PVA chains to move into sufficiently close proximity as to form the hydrogen bonds and subsequent crystalline junction points. The more freeze-thaw cycles, the tougher the gel. PVA is used in a variety of biomedical applications including drug delivery, cell encapsulation, artificial tears, artificial vitreous humor, contact lenses and more recently as nerve cuffs.
In our laboratory, we have developed a method of creating a PVA-based hydrogel through the use of solvent manipulation. By carefully altering the solution conditions of a PVA solution through the selective use of a second component, termed a “gellant,” we can drive the polymer chains into forming the crystalline junction points without a freezing process. By using biocompatible materials, the PVA solution and gellant can be injected through a narrow gauge needle into a body cavity, where it will form a hydrogel without chemical reaction or toxic byproducts. The hydrogel solution can act as a transport mechanism for therapeutic drugs or cells as well, with a structure ranging from micro to macro-pores. (See Exhibit 2 for an example of a macro-porous hydrogel.)
Exhibit 2: Scanning Electron Micrograph of Porous Structure of PVA-based Hydrogel
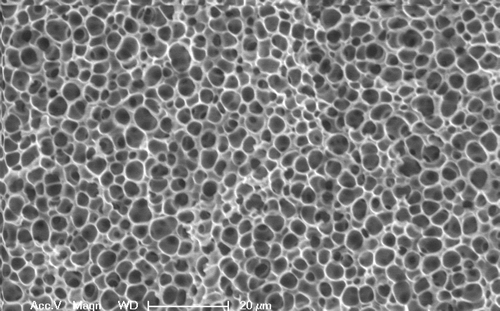

This class of hydrogels, termed “injectable hydrogels,” has found an audience for minimally invasive procedures in both orthopaedic applications and soft tissue work. Researchers have focused on hydrogel formulations derived from silk proteins, thermally-responsive phase-change materials, such as poly-N-Isopropylacrylamide, and emulsion systems in an effort to deliver a low viscosity solution into a confined body space that will then form a load-bearing, biocompatible construct.
Application Areas for Hydrogels
Arguably the largest growth areas in injectable hydrogels have been in tissue bulking, cartilage repair, nucleus pulposus replacement and scaffolding. As opposed to pre-formed hydrogels that must be polymerized in controlled conditions, involving chemical crosslinkers, injectable hydrogels for tissue bulking must inject through narrow gauge needles and gel without initially toxic monomers or crosslinkers, and also must not yield potentially toxic polymerization byproducts, all while being able to form a hydrogel in the body environment. The systems considered here are all permanent implants; degradable hydrogel structures have been developed for other applications, including tissue scaffolding and drug release.
Nucleus Pulposus Augmentation
As a case study, it is interesting to discuss the materials currently considered for nucleus pulposus replacement in the spine. The human functional spinal unit, comprising vertebrae and intervertebral disc, is responsible for both the mobility of the spine and its ability to support the dynamic loads of the human body.
However, the disc itself comprises the ply-like annulus fibrosus that contains the nucleus replacement, which is a natural hydrogel, resulting in a structure not dissimilar to the modern radial car tire. In early disc degeneration, this structure is compromised and the nucleus deteriorates, eventually resulting in chronic pain and loss of function. Thus, replacement of the nucleus early in the degenerative cascade could have a profound impact on the aging, but still active, demographic seen increasingly in the modern world.
The first attempts for nucleus replacement utilized stainless steel balls. Although this did temporarily relieve the symptoms, in the long term it resulted in end-plate subsidence and complications. This outcome highlights a key reason for attempting to mimic the materials used by the body. The steel balls supported physiological loads directly on the end-plates, whereas in reality the nucleus’s primary function is to transfer loads to the annulus and pressurize the disc. Thus, to resect minimal material (i.e. partially remove the affected joint), the replacement material must reflect the original functionality to have any hope of success. For this reason, in the late 1980s, an implantable poly(acrylonitrile)-poly(acrylamide) copolymer hydrogel encased in a polyethylene fiber jacket was developed. This device was highly hydrated and compliant, but as with all monolithic devices, it could not distribute load in the same manner as the native disc, largely relying on end-plate loading rather than pressurization and load-distribution to the disc walls. In addition, the fundamental flaw in any implantable device is that the incision required to implant it must be large enough to accommodate the device. Even if the device is dehydrated, this incision can be substantial and can ultimately result in device migration and expulsion, a problem seen in early trial implants.
Consequently, the industry has moved towards injectable nucleus pulposus replacement strategies involving protein-based materials, emulsion systems or hydrogels formed through associative bonds. All these technologies rely on an intact annulus fibrosus to transfer the compressive load in the spine from the nucleus pulposus to a radial load in the annulus fibrosus. An example of an early study conducted in our laboratory is shown in Exhibit 4 To be effective, the nucleus pulposus replacement must be able to handle ten million cycles of compression fatigue load without extrusion or expulsion, and maintain adequate disc height spacing. ASTM task groups are drafting guidance documents that address the suggested testing protocols for nucleus pulposus replacement technologies.
Exhibit 3: Schematic Diagram of Nucleus Pulposus Replacement with an Injectable Hydrogel
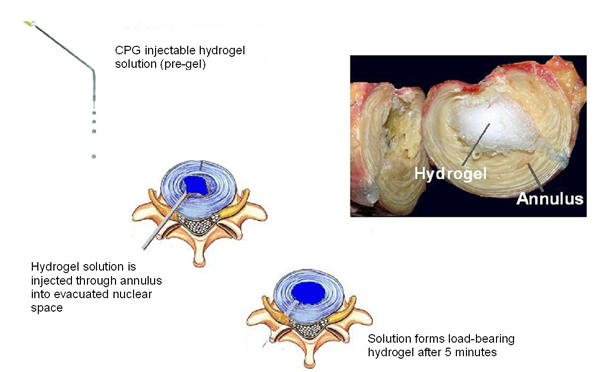

Inset (left): Solution injected through a 16G needle. Inset (right): Hydrogel implanted into a human cadaver through an annulus fibrosus injection.
Tissue Bulking
The soft tissue-like properties of hydrogels make them logical candidates for tissue bulking, or the reinforcing of soft tissue in the body with a synthetic material. Injectable hydrogels are ideal candidates for this application area. The cosmetic surgery industry has been using injectable hydrogels for several years, largely based on injectable polyethylene glycol hydrogels, to address wrinkles.More recently, injectable hydrogels have found use in addressing damaged tissue. In one example, researchers at the Massachusetts General Hospital (Dr. Judy Hung) hypothesized that ischemic mitral regurgitation can be addressed by structurally supporting the altered myocardium in patients showing displaced papillary muscles resulting from ischemic ventriculardistortion. Injections of a hydrogel developed at Cambridge Polymer Group into the myocardium of sheep with induced mitral regurgitation demonstrated acute reverseremodeling of the ventricle with papillary muscle repositioningto decrease mitral regurgitation. In another example, we are investigating hydrogel injections to address urinary incontinence.
Tissue Models
Uses of hydrogels beyond permanent implants have already been realized, with the increasing use of tissue models derived from hydrogels for device testing and training. The biomimetic properties of hydrogels allow the casting of realistic and reproducible tissue models, thereby delaying or avoiding the need for animal testing. Examples of some models developed in our laboratory are shown in Exhibit 4.
Exhibit 4: Hydrogel-based Tissue Models


Shown: Vascular models (left) and a subcutaneous fat model (right).
Conclusions
There is a further hurdle that soft-solids, and hydrogels in particular, must pass before they can be considered viable for biomedical devices: relevant pre-clinical testing. Almost all of the potential applications for hydrogels involve working in conjunction with the surrounding tissue.
Unlike conventional biomedical materials, like polyethylene on metal in hip replacements, the functionality of these soft-solids is largely dependent upon their interaction with the surrounding tissues and the viscoelastic nature of those tissues. This requirement places significant challenges on how these materials should be tested. In the specific case of the nucleus replacements, in which the material is intended to operate in conjunction with moderately intact end-plates and annulus fibrosus, simple biomechanical testing of the free-standing nucleus (tension, compression, etc.) is irrelevant; instead, a testing environment that mimics the confinement of the nucleus is important, not just for mechanical properties, but also for fatigue and wear.
Surgeons and engineers are increasingly looking to hydrogels to solve complex physiological problems that cannot be easily addressed using conventional approaches. Even if the material cannot provide cradle-to-grave use and will eventually wear out, quality of life will be greatly enhanced by providing a stop-gap outpatient treatment early in the patient’s life before more invasive surgeries are required. Hydrogels in orthopaedics have only begun to reach their potential, and although they will never completely replace current designs, they give the potential for an important alternative strategy to maintaining patient health and mobility.
Jason Berlin holds a B.S. and M.S. in Biomedical Engineering from Boston University. His Masters’ thesis involved hydrogel synthesis and characterization for cartilage and cornea repair applications, and his research at Cambridge Polymer Group (CPG) is focused on characterization methods for biomimetic materials, custom formulations and tissue work. Jason has held positions at DePuy Spine and Sontra Medical, and is currently a primary investigator on two NIH-funded projects developing hydrogel technology for nucleus pulposus replacement and tissue bulking in the urinary tract. He also performs consulting and research on polyethylene orthopaedic implants.
Gavin Braithwaite, Ph.D. is Vice President of Research at CPG. He received his B.S. in Physics from Edinburgh University, his M.S. in Electrical Engineering from Southampton University and his Ph.D. in Chemical Engineering from Imperial College. He was a post-doctoral fellow at Harvard University and the Massachusetts Institute of Technology. Dr. Braithwaite holds several patents on hydrogel formulations, analytical instrumentation and natural polymers. At Cambridge Polymer Group, Dr. Braithwaite is in charge of sponsored research programs as well as CPG’s internal research efforts. He is also heading a new division at CPG involving device design and validation.
Stephen Spiegelberg is President and co-founder of CPG. He received his B.S. in Chemical Engineering from the University of Wisconsin-Madison and his Ph.D. in Chemical Engineering from the Massachusetts Institute of Technology. He was a post-doctoral fellow at Harvard University. He holds patents in analytical instrumentation and materials for biomedical application. At CPG, he directs a team of scientists performing contract research and testing on polymeric materials for the biomedical community and other fields. He chairs task groups in ASTM on the cleanliness of biomedical devices, medical device shipping and characterization methods for thermoplastics.
Cambridge Polymer Group, Inc.
REFERENCES
1. Peter Venkman, Ghostbusters II, Columbia Pictures, 1989.




