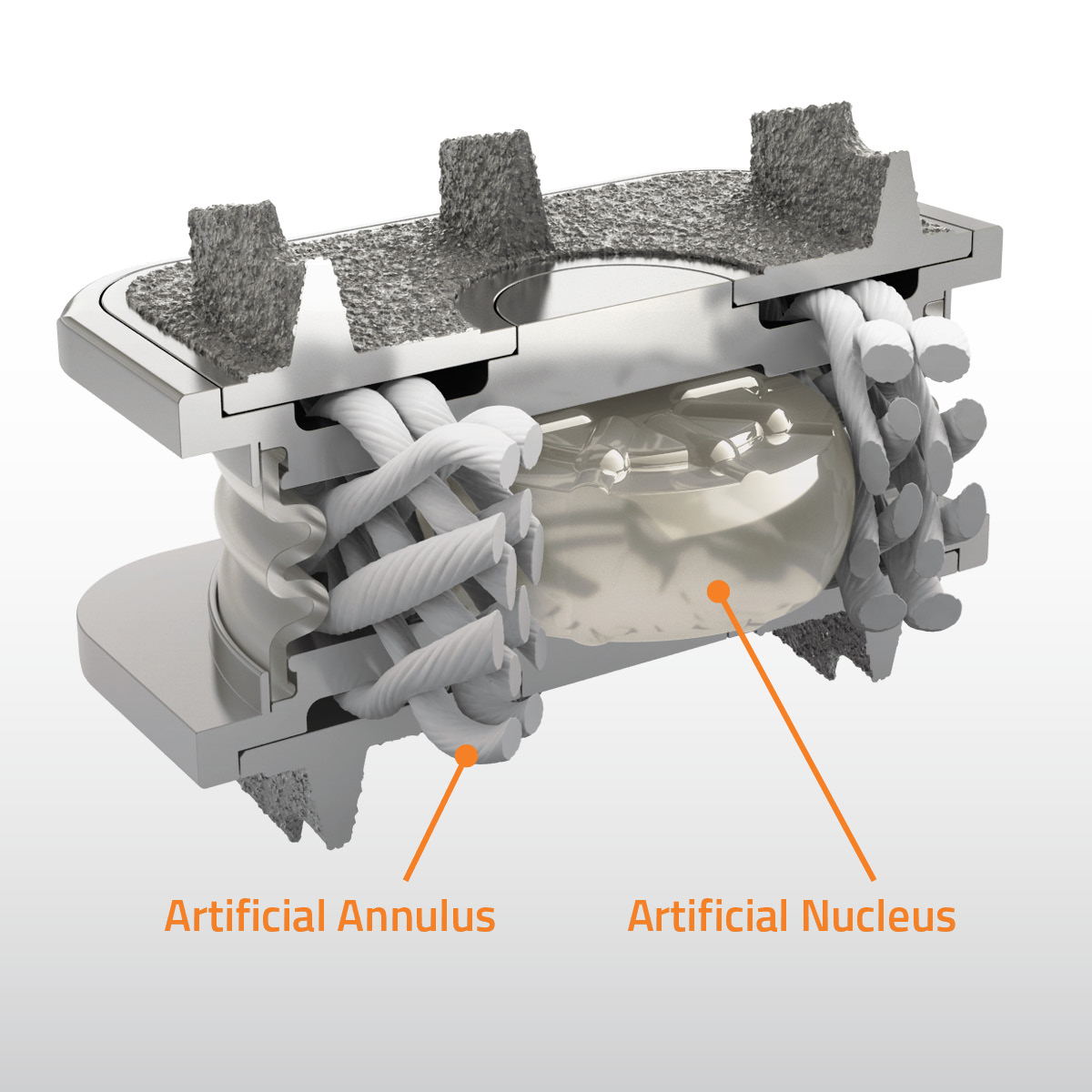
Orthofix Medical announced full 2-year outcomes from its U.S. Investigational Device Exemption study of the M6-C™ artificial cervical disc vs. anterior cervical discectomy and fusion (ACDF).
Results from the prospective, non-randomized, concurrently controlled clinical trial indicate that patients treated with M6-C had significant improvements in pain, function and quality of life. Further, at 24 months, patients in the ACDF group who were still using pain medications had a seven times higher rate of opioid use than those who received M6-C.
Other study findings included:
- A statistically significant difference in the average mean surgery time – 74.5 minutes for M6-C procedures vs. 120.2 for ACDF
- A statistically significant difference in the mean length of hospital stay – 0.61 days for M6-C patients vs. 1.10 days for ACDF patients
- Lower subsequent surgery rates at the treated level – 4.8% of ACDF patients compared to 1.9% of M6-C disc patients
- No device migrations reported
- A 92% patient satisfaction rate with M6C
The M6-C disc received FDA Premarket Approval in 1Q19 based on the results of this study.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




