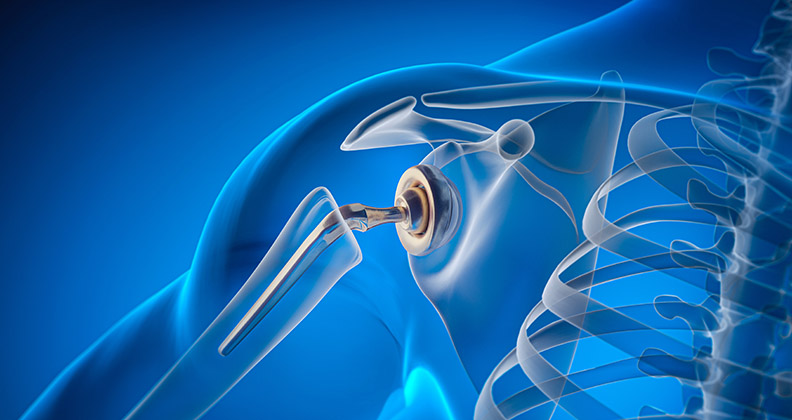
Device companies continue to respond to surgeons’ requests that when it comes to instruments in the O.R., in some instances, less is more. When the procedure allows for it, OEMs are reducing their number of instruments to make the surgery more predictable, efficient and cost-effective, which can yield less money and time spent on sterilization and storage—all considerations appropriate for both hospital and ASC settings.
We realize that the concept of reduced instrumentation is certainly not a new one, and that many device companies are designing implants and systems with this consideration in mind.
For instance, these companies are combating the cost associated with instrumentation in operating rooms. They have capitalized on the shift toward simpler procedures with fewer instruments with recent product launches or revisions, process initiatives and 510(k) clearances.
- In August, ConforMIS announced completion of the first two iTotal Hip replacement procedures. The system is delivered directly to the hospital or outpatient facility in a single patient-labeled kit, eliminating the need for excess inventory. Patient-conforming, single-use, 3D-printed cutting guides are included. Timing for a complete commercial launch will be announced in 2019.
- DJO Global received FDA 510(k) clearance in 2017 for the Exprt® Revision Hip, designed with a two-tray system that represents an 80% to 90% reduction in instruments vs. competitive systems. DJO also features this two-tray system as part of its Exprt® Revision Knee. DJO leadership commented to BONEZONE that the two-tray system was a response to surgeon feedback that the Exprt Knee procedure was too complex. As surgeons attained more experience doing revisions, they used fewer instruments.
- Exactech’s Equinoxe Stemless Shoulder, currently in limited launch stage, is accompanied by a single instrument tray to support O.R. efficiency.
- Flower Orthopedics launched new instruments in the Flower Efficiency (E-Kit™) Advanced system in 3Q17: FlowerGraft™, a foot reconstruction platform with pre-shaped, bi-cortical allografts packaged in saline; and the addition of Headless Compression Screws to the Ready-for-Surgery™ cannulated screw set. One E-Kit can reportedly replace the instruments in two sterilization trays.
- Study results announced in 2017 indicated that Johnson and Johnson Medical Device Companies’ (JJMDC) CareAdvantage Perioperative Efficiency approach yielded improvements for UCSF Health hip and knee procedures, such as reductions in instrument trays (down 57%), number of instruments used (down 29%) and number of open trays (down 46%), leading to an estimated annual cost savings of >$262,000. The CareAdvantage process identifies the customer/health system’s needs and objectives pertaining to the delivery of value-based care. This information feeds into an action plan that includes metrics to measure results. The program helps to streamline the path of JJMDC devices through surgical services and sterile processing.
- Meditech Spine’s CURE Lumbar Plating system and Talos®-A fusion device were recently combined into one tray, with streamlined instruments, to improve ease of processing for hospitals and ASCs.
- NEO Medical’s NEO Pedicle Screw System, which received FDA clearance in 2017, replaces a typical instrumentation set of seven trays and 60+ instruments with five instruments. The system weighs just over two pounds and optimizes 200+ screw sizes and designs into 14 screws. The five core instruments include an awl for breaking the cortical bone in open surgery, a steffee to measure which screw is needed, a screwdriver and torque handle, and a rod holder for use during minimally invasive surgery.
- SeaSpine’s Mariner® pedicle-based system for posterior spinal fixation, launched in 3Q17, is designed to reduce the number of trays required in the O.R. Mariner features screws with in situ modularity and accompanying instrumentation for the treatment of degenerative and complex spine pathologies.
- In 2016, United Orthopedic Corporation President Calvin Lin told BONEZONE that the company’s U2™ Knee System has been revised since initial launch with reduced instrumentation in mind. “By redefining our instruments and using more disposable trials, we were able to significantly reduce our instrumentation,” Lin said. You can read more of Lin’s comments on reduced instrumentation here.
Rob Meyer is ORTHOWORLD’s Senior Editor. Please send comments on this article to Carolyn LaWell.