Your company is ISO 13485 certified and has internal processes in place. Individual departments have well-established SOPs. Yes, you have successfully introduced several products to market on a project-by-project basis. If it’s not broken, why fix it? Why the need to integrate strategies?
“Strategy Integration” has emerged as the new catchphrase in the medical technology sector. As companies face daily challenges of bringing new products to market, they continuously seek ways to drive efficiency, reduce internal costs and expedite product approvals to drive profitability.
In addition to the daily operational activities, successfully launching a product requires the combined efforts of several key functional groups: regulatory affairs, clinical research, reimbursement and marketing. The Regulatory team has to navigate the maze of FDA; Clinical Research is tasked with satisfying enrollment in a timely manner while producing data to meet the needs of key stakeholders, Reimbursement will grapple with the Federal healthcare system for payment constrained within a cost-contained environment, and Marketing has to create a value proposition to produce the return on investment that shareholders expect from forecasted sales.
In the end, the individual strategies of each functional group will impact the others. If the individual strategies are integrated early in the product development phase, the better the chance of a timely, successful launch. The clinical trial protocol will impact FDA clearance. The approved labeling will dictate what is medically necessary for payment. The target market will be determined by who will pay and who will buy. Yes, the needs of FDA, payers and the market are different, but are all very much aligned.
A well-designed, departmentally-integrated strategic plan (a.k.a. your Blueprint) will drive efficiency, decrease time to market and ensure coverage and payment, while driving market adoption and generating revenue, thus increasing company profitability. We see our business change day to day as a result of many uncontrollable factors in the healthcare environment. It is important to be as flexible as possible with your Blueprint.
There is no one right way to build the strategy; however, several key components are necessary to build the foundation of the plan.
Critical Components of the Blueprint: Your Roadmap to Success
Product Development Phases
Phase I: Conceptualization – The product idea is conceived.
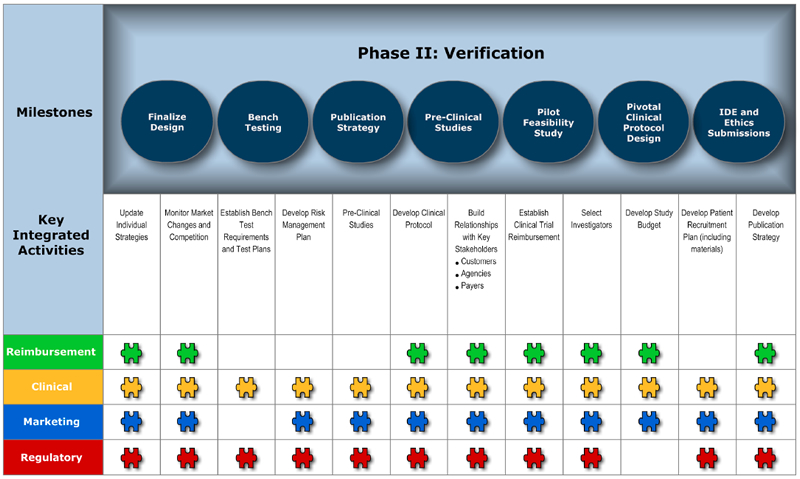
Phase II: Verification – Determining whether the product can be manufactured.
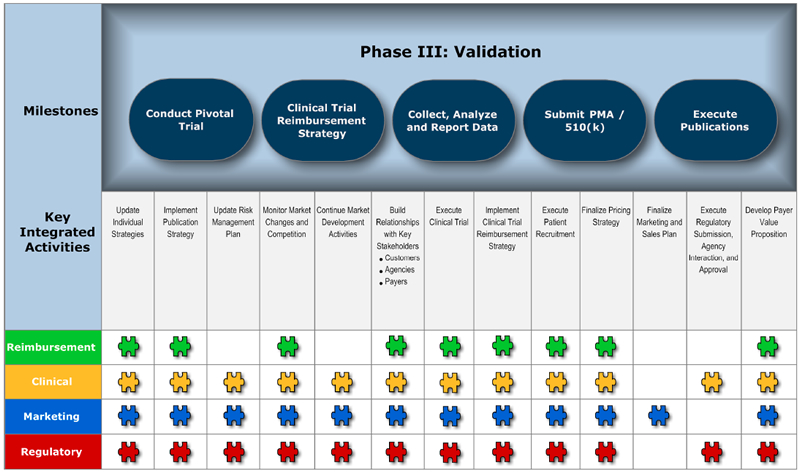
Phase III: Validation – Is the product reproducible, marketable, will it get approved?
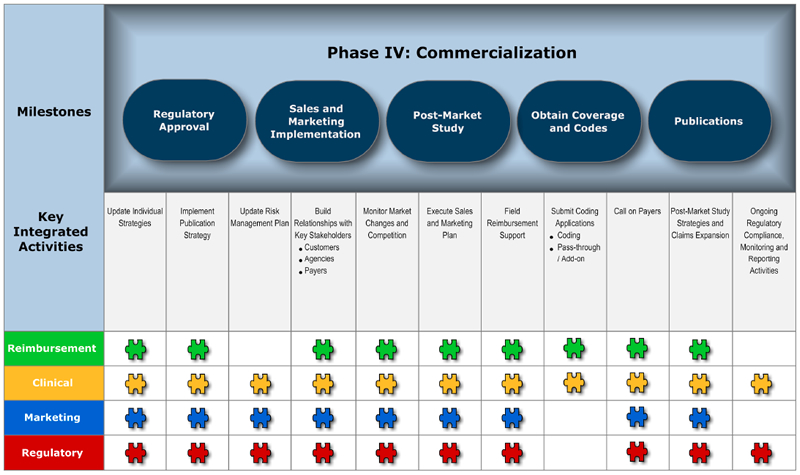
Phase IV: Commercialization – Will the product sell? To whom, and for how much?
Milestones
Milestones do not belong to the individual groups represented, but to the entire organization. Identify what you want to achieve within each product development phase and agree on ownership. It is also critical to put metrics into place to track and analyze the success of the project.
Key Integrated Activities
These key activities will support the identified milestone in each phase. Two, three or four key functional groups may be involved in an activity. Keeping in mind that the Blueprint is a flexible process, you may need to involve other key functional groups to successfully complete the activity.
Analyzing Success
After completion of the project, it is important to review the process and determine what was effective, what wasn’t and what can be changed to ensure a higher success rate for the next project.
Tying It All Together
Charts 1-4 depict the four phases of product development, including key milestones, key integrated activities and functional groups involved in the successful launch of a medical device.
Integration will have a different meaning to every department and every company. However, the underlying message is simple. By identifying the appropriate milestones, involving the appropriate key functional groups, creating realistic milestones and maintaining flexibility along the way, companies can successfully create and implement an integrated plan without the process becoming too complex. The end result will be a new process for future projects that will not only ensure a successful product launch, but also drive efficiency, reduce internal costs, expedite product approval and most importantly, have a positive impact on a company’s bottom line.
Kelli Hallas is the Executive Vice President of Reimbursement at Emerson Consultants, Inc., with over 20 years of experience in the Medical Device sector.