
Primary total hip arthroplasty (THA) has undergone 25 years of product development, thanks to contributions from a host of materials technologies. The result? A reliable, fully-functional, pain-free, lifetime hip replacement for most patients. Using today’s lexicon, this now-routine procedure has been succinctly termed the “forgotten hip.”
As is common in all areas of human progress, the road to the forgotten hip has been paved with certain pitfalls. One example is Sir John Charnley’s discovery of high density polyethylene in the early 1960s as a suitable material for the acetabular bearing, after learning that PTFE (Teflon) was not the solution. The success of THA depends upon many factors including design, surgical technique, post-operative care and materials, with the latter arguably being the most influential in realization of the forgotten hip today and in the future, for most primary THA patients.
Before we predict advancements, let’s review how we arrived at the current standard for THA.
Evolution of Fixation
In the early 1990s, cementless primary hip arthroplasty was rapidly supplanting cemented arthroplasty with polymethyl methacrylate, or PMMA, as the majority means of fixation. This shift can be traced back to the 1980s with the introduction of new thermal spray and sintered coating technologies, ranging from “large” beaded coatings most notably promoted by Howmedica, “small” sintered beads marketed by DePuy, fiber metal from Zimmer, hydroxyapatite from Osteonics and plasma sprayed titanium from Biomet.
Subsequently, all companies were offering one or more solutions for cementless fixation by the mid-1990s. (See Exhibit 1.) These surface modifications all had one primary purpose: to provide fixation of femoral and acetabular components to bone. The generally accepted range for porosity of the porous coatings for bone ingrowth was between 250 to 750 micrometers, which still holds true today.
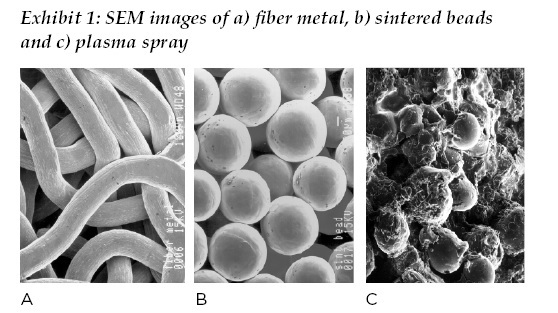

During this time, engineers and clinicians alike learned the importance of friction between the implant surface and bone in establishing initial stability with a cementless implant, the volume porosity and depth of porosity available for bone ingrowth and maintaining biological equilibrium, the engineering compliance between bone and implant surface in terms of stiffness /modulus of elasticity, and biomechanical loading of the hip and relationship to bone remodeling.
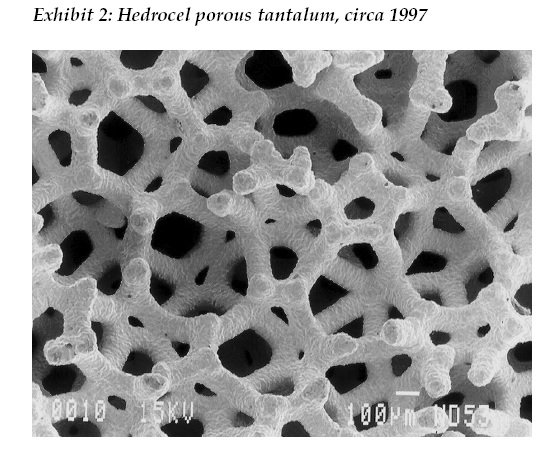
Beginning in 1997 with Hedrocel porous tantalum and later trade named Trabecular Metal by Zimmer, three dimensional, porous structured titanium and tantalum materials were introduced to orthopaedics. (See Exhibit 2). Since then, with 20 years of documented clinical success in primary and revision hip arthroplasty, the state of the art for cementless fixation to bone for primary hip replacement implants are three dimensional, porous structured surfaces manufactured by chemical vapor deposition, sintering, traditional foaming and additive manufacturing (3D printing), with the latter method being available to any company willing to adopt the technology. And let us not forget cemented hip replacement, the reliability and survivorship for which has greatly increased since the 1960s due to improved formulations and methods of use. In turn, mechanical properties of polymethyl methacrylate (PMMA) became available with antibiotics (e.g. gentamicin) in the 1980s in Europe and in the early 2000s in the U.S.

The Quest for Lifetime Bearings
The 1990s in orthopaedics were notable, or maybe better said, notorious, for the emergence of osteolysis and premature implant loosening as major issues, caused by high rates of polyethylene wear. The adverse clinical and biological effects were clear, with consequent decrease in implant survivorship and uptick in revision hip arthroplasty.
Academic and industry research into the cause(s) of high rates of wear led first to the understanding of oxidative degradation of polyethylene and increased wear, and cross linking and decreased wear, due to gamma radiation sterilization in air (at the time). Further research into the issue by industry led to better understanding, and in turn control, of the process parameters of time, temperature and pressure in consolidating polyethylene resin into rod and sheet forms, along with consequent effects on the mechanical, tribological and stability properties over time. This research led to the second generation of polyethylene materials use in THA, which included gamma radiation sterilization of polyethylene performed in inert gas or vacuum packages (e.g. Biomet’s ArCom), elimination of gamma radiation altogether (e.g. Smith & Nephew and Wright Medical) and higher quality polyethylene being produced by the suppliers (e.g. PPD Group, Orthoplastics) to the manufacturers.
Concurrently, the orthopaedic industry was renewing its interest in metal-on-metal and ceramic-on-ceramic hip bearings, which were used more frequently as companies sought to eliminate osteolysis and early loosening caused by polyethylene wear. The most commonly available, trademarked ceramic product of the time was Ceramtec’s BIOLOX alumina; Smith & Nephew’s Birmingham hip is the most noteworthy, long-term successful MOM device on the market today.
Starting in 1999, third-generation polyethylene materials began to enter the market, characterized by moderate levels of cross linking via electron beam and gamma radiation methods, coupled with post cross linking, thermal processes to reduce or eliminate free radicals (that cause oxidation). Third-generation materials of wide notoriety include Zimmer’s Longevity and DePuy’s Marathon. Starting in the mid-2000s to this day, fourth-generation polyethylene materials are characterized by high levels of cross linking (75 to 125 kGy radiation) and vitamin E for stabilizing the material against oxidation. The published research and early clinical results with vitamin E, cross-linked materials suggest that this fourth-generation polyethylene can last “a lifetime” for high-demand THA.
The long road to the fourth-generation polyethylene was characterized by voluminous clinical and scientific research into the problems and solutions, with numerous ASTM consensus standards (F648, F2003, F2102, 2183, 2381, 2565, 2625, 2695, 2759 and F2848) and an FDA guidance document (“Characterization of Ultrahigh Molecular Weight Polyethylene (UHMWPE) Used in Orthopedic Devices”) developed during this time. Collectively, these standards for testing and analysis provide reasonable insurance against the industry repeating the mistakes of the late 1980s and early 1990s regarding polyethylene-based hip bearings.
In parallel with the transition to highly cross-linked polyethylene, the past 25 years have been characterized by significantly increased use in ceramic/ceramic hip arthroplasty, with that increase persisting today. The past few years have also seen an increase in ceramic/polyethylene articulation, in large part due to the concern of fretting/corrosion of CoCrMo alloy/titanium alloy modular junctions. Also dating back to the late 1990s is increased use of metal-on-metal articulation, that unlike ceramic, has fallen to pre-1990s levels due to factors not strictly related to the composition and tribology of CoCrMo hip bearings, as evidenced by successful clinical results of some companies’ systems.
What Will THA Look Like in 2042?
Given the past 25 years of progress in biomaterials applied to THA, what does the future hold? Given the inherent, incremental evolutionary change in the practice of orthopaedic surgery, and the topics of the day at American Academy of Orthopaedic Surgeons (AAOS) and American Association of Hip and Knee Surgeons (AAHKS) including infection, early healing/recovery and learning from recent mistakes in design and materials, we can expect the following:
Materials Focus
- Polyethylene will continue to be an option for the acetabular bearing, with further technological advances in resistance to wear and degradation.
- Ceramics, CoCrMo and coated metallic materials will continue as options for the femoral head, with reductions in cost due to advances in technology and processing equipment. Titanium alloy will remain as the most common material for the femoral hip stem component.
- New ceramic materials will be developed that reduce the risk of fracture of femoral heads. Also, new equipment and manufacturers will help decrease the cost of ceramic heads.
Resurfacing Solutions
- New high-strength, more natural synthetic cartilage/tissue replacement solutions will make inroads into resurfacing hip arthroplasty.
- Synthetic chemical changes/surface engineering will increase the rate of tissue healing for establishing higher strength of early fixation of implants to bone.
- Surface chemistry modification and incorporation of drugs to reduce potential for infection will become common place.
Design and Manufacturing Processes
- We will see the reemergence of composite materials for the femoral hip stem and innovative designs using existing materials that better mimic the biomechanics of the femur, while maintaining fixation of the device to bone.
- Additively manufactured products with structured porous surfaces for bone ingrowth and fixation will become commonplace.
- Additively manufactured, disposable/recyclable instruments will also become commonplace.
While a purely biological solution to hip replacement is an ideal goal for hip replacement—call it the “utopian hip”—surgeon, industry, regulatory and technology factors will continue to dominate incremental progress in materials applied to THA, with patient heredity, behavior and lifestyle leading to device-based solutions for the foreseeable future.
Robert A. Poggie, Ph.D., is President of BioVera. His previous employers and functions include Smith & Nephew, Implex, Zimmer and Pipeline Orthopaedics, with responsibilities in applied research, biomaterials, clinical research, medical education and regulatory affairs.
Images courtesy of the author.




