Advances in the development of prosthetic joints and materials have led to a need for more effective testing solutions. Testing for and proving robustness is of particular importance because of the risks, inconvenience and costs involved with revision surgery and associated litigation. Further, regulators are expecting additional testing to be performed and standard methods are being written, so many manufacturers are pre-empting the questions and performing them as standard, along with other analyses such as debris analysis and surface roughness.
Traditional wear testing of articulating joints simulates the motion and loading that a typical implant is expected to undergo in vivo. Components are weighed before and after a run cycle to determine any change caused by wear of the device. Simulations run for months, typically over five million cycles.
Wear testing of hip and knee implants is a regulatory requirement for ISO and ASTM standards. Conditions used during wear testing are idealized and do not reflect the full range of forces to which the implant will be exposed. Therefore, testing for adverse events such as impingement, third bodies and microseparation has its advantages.

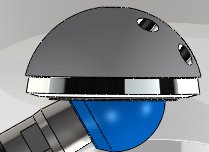
A femoral stem at the limit of its natural motion. When motion cycles are applied in this test, the femoral stem will impinge on the acetabular cup.
Impingement Testing
When the articulating components of the implant extend beyond their natural motion, they will impinge upon the joint, as illustrated in Exhibit 1.
Impingement testing pushes the hip prosthetic beyond normal limits, as may be experienced in vivo, especially if the patient remains active following joint replacement. The test is performed under lower load limits, as an impinged joint is unlikely to experience maximum force. The joint is then tested through a series of cycles of defined load and motion.
Impingement tests are monitored for the generation of additional wear. This is achieved by running simultaneous control samples which are set at the “standard” angles outlined in ISO 14242 so as not to impinge during the motion cycle. Impingement could generate wear debris, wear tracks or even fracture of the components, all of which have the potential to lead to joint dislocation, causing significant pain and sometimes revision surgery. An observational study found that 60% of hip replacement recipients suffered from some level of impingement; of those, one-third were considered to be severe impingements. It is important to consider such factors when designing and developing better prosthetics for joint replacements.
Third Body Testing
Third body testing refers to the presence of foreign materials within the closed membrane that encapsulates articulating joints. The natural membrane contains synovial fluid that provides nutrients for cartilaginous tissue, cushioning for the joint and lubrication for the surfaces. During joint replacement, this membrane has to be opened to gain access to the joint and it is extremely likely that third body components will enter the fluid, as well as wear debris from the implants themselves.
To simulate the effects of third body wear, simulations are run with either standard or impingement configurations. Potential third body contaminants are then added to the bovine serum mixture, as determined by the implant manufacturer or literature. Test contaminants tend to include bone cement particles (a common contaminant during replacement surgery) or metal/ceramic particles of known size, which could be generated by wear of implant components.
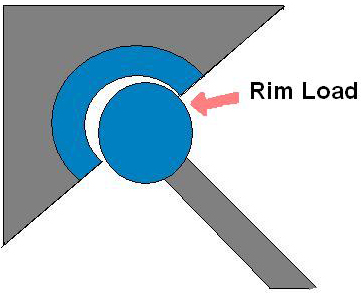
The femoral head has dislocated as it overextends, and the rim load is highlighted on the edge of the acetabular cup.
Microseparation Testing
Microseparation of the knee and hip joints occurs clinically at the swing phase of the gait cycle, where the joint laxity is greatest and the load is minimal. This results in over extension of the joint, allowing the femoral head to dislocate, as can be seen in Exhibit 2, and make contact with the edge of the acetabular cup. Over-extension can result in wear at the contact point between the femoral head and acetabular cup edge.
The effect of microseparation is simulated by medial translation of the articulating joint, so that there is localized contact with the stationary component. Motion and load are simulated to replicate in vivo conditions and the process is repeated through a number of cycles, either specified by relevant standards or by the implant manufacturer. The implant can be removed at intervals to assess the wear rate and development of wear tracks.
Why Consider Adverse Event Testing?
Adverse event testing takes into account some of the worst case scenarios that an orthopaedic implant may experience. Performing adverse event wear testing during the development stage allows implants to be designed to reduce the effects of, or overcome, these issues.
By considering all of the requirements of the implant, manufacturers can create products that improve upon those currently on the market and offer better performance for the patient.
Gemma Budd is Healthcare Business Manager at Lucideon.
She can be reached by email.
Photos Courtesy of Lucideon
Lucideon, an industry expert in materials testing, analysis and consultancy, published a white paper on this subject in September 2015, titled “The Advantages of Including Adverse Event Wear Testing in the Design of Orthopedic Implants.”




