Share on:
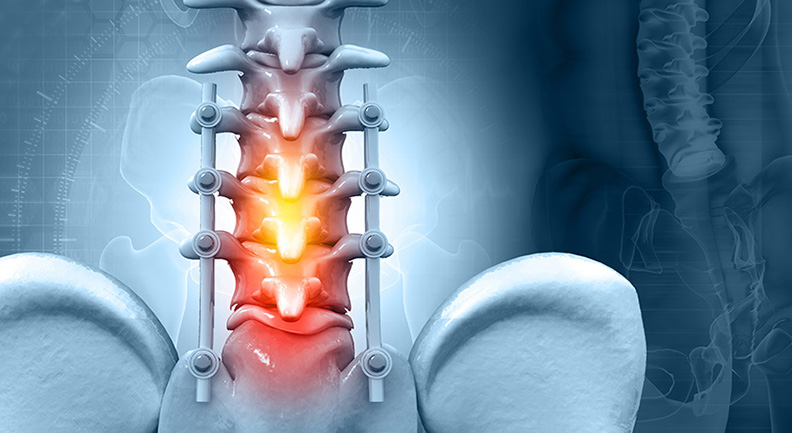
FDA cleared the Dyneema Purity® Radiopaque Cerclage Cable for use in orthopaedic trauma treatments, such as bone and spine fractures. This marks the first clearance for a device fully designed and developed by DSM’s Biomedical Polyethylenes Team.
Purity Radiopaque fiber is reportedly 15 times stronger than steel, with high pliability and flexibility that may help lower the risk of bone damage. The fiber is biocompatible and chemically inert, which can help reduce tissue inflammation, irritation and complications associated with metal allergies.
Source: DSM
RELATED ARTICLES
Janco Medical to Focus on Packaging and Cleanroom Manufacturing
Apr 10 2024 , Julie A. Vetalice
Evonik Grows Capacity for Customized RESOMER Powder Biomaterials
Apr 10 2024 , Julie A. Vetalice
Exploring Why Automation Leads to Improved Medical Device Manufacturing
Apr 09 2024 , Lowell
FDA De Novo for Orthobond’s Ostaguard Antibacterial Surface Treatment
Apr 08 2024 , Julie A. Vetalice