
FDA’s recently-released final guidance documentation for 510(k) submissions remains a hot topic for the orthopaedic industry. The final guidance attempts to clarify FDA’s substantial equivalence requirements. In reality, the document doesn’t provide a groundbreaking update. However, FDA does outline its recommendations on what needs to be submitted for your 510(k)—specifically, the process by which reviewers make substantially equivalent determinations.
In a 510(k) submission, you are trying to demonstrate that your device is substantially equivalent to another device already on the market. FDA then makes an assessment of the risks and benefits in a comparison between the devices. What are the key takeaways from the guidance document?
Predicates
In a 510(k), also known as a Premarket Notification, you, as the submitter, identify one or more predicate devices in which you suggest to FDA that your device is at least as safe and effective as a device already on the market with the same intended use. It is not uncommon to identify multiple predicates, and one strategy to gain clearance utilizes a term coined as “split predicates.”
In the case of split predicates, you compare your device to one predicate that has the same intended use and to a different predicate that has similar technological characteristics. You are attempting to demonstrate substantial equivalence on an unfounded basis, akin to combining different attributes of more than one device into a single, nonexistent predicate.
FDA is clarifying that this strategy is inconsistent with the 510(k) regulatory standard. One high-profile event that illustrates the pitfalls of using split predicates is the recall of the DePuy ASR XL Acetabular Cup System metal-on-metal hip implant. As discussed in an article in The New England Journal of Medicine, “the device had three characteristics uniquely combined in the ASR XL, but were evaluated for substantial equivalence by comparing select characteristics to different predicate devices, none of which contained all of these characteristics, i.e. they were split predicates.”
Additionally, the Agency is asking that submitters identify a primary predicate in their 510(k)s. The primary predicate is one with indications for use and characteristics most similar to your device. The guidance does, however, still allow for the use of multiple predicates. This would be relevant when you are combining features from two or more predicates with the same intended use into your device or when your device has more than one intended use. The submitter would still need to make appropriate comparisons between your device and each predicate. Alternatively, you can also identify reference devices that direct attention to similar situations seen in the past.
Indications for Use and Intended Use
These two terms are often used inconsistently or interpreted differently among people. Intended use describes the purpose or function of the device, and indications for use describes the disease or condition the device will treat (including intended patient population). The intended use encompasses indications for use. To show substantial equivalence, your device’s indications must fall under the predicate device’s intended use.
FDA explains when different indications for use would preclude a predicate comparison and precipitate a new intended use. In determining substantial equivalence, the reviewer may have concerns that 1) FDA did not see with the predicate or 2) the predicate cannot be generalized to include the new indications of your device due to a “probable, significant change in the incidence or severity of the issue.”
The guidance documentation provides examples of changes that may require a new intended use:
– Changes in patient populations (e.g. adult vs. pediatric)
– Changing an indication’s anatomical structure of use
– Changing a functional indication to a treatment indication
– Changing a diagnostic indication to a screening indication
– Changed clinical contexts or settings
A new intended use would result in a not substantially equivalent (NSE) decision for your device, although this is a relatively rare reason for NSE findings.
Substantial Equivalence Pathway
Once FDA has determined that your identified predicate device(s) is (are) acceptable, the reviewer will next compare the technological characteristics in order to assess the risks and benefits. Technological characteristics are details such as material, design, energy source (if applicable), density, porosity, degradation characteristics and other features.
After the identification of the characteristics, the reviewer will assess the differences between your device and the predicate device(s). If there are any differences, the reviewer will determine if your device raises concerns of safety and effectiveness. Exhibit 1 illustrates the overall decision making flowchart from the guidance document.
Exhibit 1: 510(k) Decision Making Flow Chart as Adapted from FDA Guidance
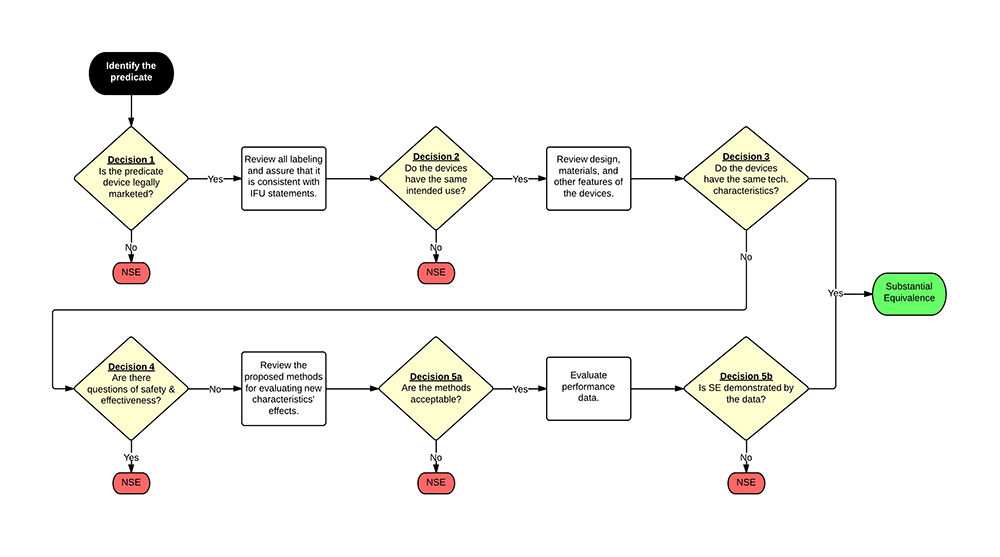

(Click on the image for a larger view.)
NSE = not substantially equivalent
IFU = indications for use
FDA explains that this document was created to enhance the predictability, consistency and transparency of the 510(k) premarket review process. By updating the definition of substantial equivalence, use of predicates and determination pathway, the Agency is trying to make the process easier for both the reviewer and submitter. Sometimes, even the simplest of ideas can have a rocky road when not considering the regulatory pathway for device clearance. Considering and complying with FDA recommendations early in the development process will help you bring the best idea to market faster.
Justin Rowland is an engineer at Kapstone Medical, LLC, a medical device engineering and consulting firm, where he helps manufacturers and surgeon inventors develop and commercialize new devices. He can be reached at jrowland@kapstonemedical.com or through LinkedIn.
Kapstone Medical
www.kapstonemedical.com




